- The First Partnership on NTDs between the WHO and a Japanese Corporation
- Participation in the Global Public-Private Partnership “London Declaration” to Combat NTDs
- Eisai-Produced DEC tablets Became the First Drug for NTDs Worldwide to Receive the WHO Prequalification
- Started manufacture of DEC tablets at the Vizag Plant in India and Commenced Free Supply to the WHO
- Participation in Partnership to Provide Diagnostic Kits for LF
- Fifth Anniversary of Public-Private Partnership “London Declaration”
- World NTD Day
- Endorsing the New Public-Private Partnership “Kigali Declaration” to Combat NTDs
- Current Distribution Status of DEC Tablets for LF Endemic Countries
Eisai aims to effectively achieve social good in the form of relieving anxiety over health and reducing health disparities under its human health care (hhc) concept. One social good that we are focusing on is the elimination of neglected tropical diseases (NTDs), which is a target (3.3) of the United Nations’ Sustainable Development Goals (SDGs). Eisai has been engaged in the elimination of lymphatic filariasis (LF), one of the NTDs, since 2010. The World Health Organization (WHO) conducts mass drug administration (MDA) campaigns in endemic countries to eliminate LF. Diethylcarbamazine (DEC) tablets, one of the LF treatments, was in short supply globally, and this posed a serious obstacle to the elimination of LF. Eisai is committed to manufacturing and supplying high-quality DEC tablets free of charge through the WHO and is engaged in various activities to eliminate LF.
Animation video introducing Eisai’s 10-year efforts to combat LF
Eisai’s Initiatives to Combat LF
Eisai’s Initiatives to Combat LF
2010
The First Partnership on NTDs between the WHO and a Japanese Corporation
Eisai signed a joint statement with the WHO in which it committed to supplying DEC tablets, a treatment for LF, to the WHO at price zero. It was the first partnership formed by a Japanese company with the WHO to provide a medicine for NTDs free of charge. In the joint statement, Eisai agreed to develop and manufacture high-quality DEC tablets and provide a total of 2.2 billion to the WHO free of charge by 2020. Please click here for more details.
In 2017, Eisai announced to provide DEC tablets to endemic countries that need them until elimination in achieved in these countries.

Dr. Margaret Chan, Director-General, WHO and Dr. Haruo Naito, CEO of Eisai
2012
Participation in the Global Public-Private Partnership “London Declaration” to Combat NTDs
Together with twelve other global pharmaceutical companies, the Bill & Melinda Gates Foundation, the WHO, the U.S. and U.K. governments, the World Bank, and governments from NTD-endemic countries, Eisai pledged its support to the London Declaration, a coordinated effort to eliminate NTDs, as the only Japanese company. Please click here for more details.

(Dr. Margaret Chan, on the far left, Mr. Bill Gates, Co-chair, the Bill & Melinda Gates Foundation, in the center, and Dr. Haruo Naito, CEO of Eisai, on the far right)
2013
Eisai-Produced DEC tablets Became the First Drug for NTDs Worldwide to Receive the WHO Prequalification
Eisai-produced DEC tablets were officially recognized as meeting the WHO’s stringent standards by receiving WHO prequalification. This was the first case in the world where a drug for the treatment of an NTD was prequalified by the WHO.
Started manufacture of DEC tablets at the Vizag Plant in India and Commenced Free Supply to the WHO
Eisai began providing DEC tablets produced at its Vizag Plant in India to the WHO free of charge. The initial shipments of DEC tablets were sent to four countries—Papua New Guinea, Kiribati, Tuvalu, and Fiji.

In addition to the free supply of DEC tablets, Eisai is engaged in multifaceted approaches to combat LF including support for the implementation of MDA campaigns, disease awareness activities and infrastructure improvement.


Please click here to see our latest articles about local activities
Eisai's Challenge of Greater Access to Medicines:
Tackling Lymphatic filariasis (video)
2015
Participation in Partnership to Provide Diagnostic Kits for LF
Eisai has participated in a public-private partnership with the WHO, the Bill & Melinda Gates Foundation and two other pharmaceutical companies to provide diagnostic kits free of charge, for use in evaluating the success of MDA in LF elimination. Please click here for more details.

2017
Fifth Anniversary of Public-Private Partnership “London Declaration”
Donor Companies Including Eisai Set a Guinness World Record for “Most Medication Donated in 24 Hours”

In the 5th anniversary event of the London Declaration, Eisai announced it would continue to provide DEC tablets, a treatment for LF, via the WHO free of charge to endemic countries that need DEC until complete elimination of LF is achieved.

To commemorate the 5th anniversary of the London Declaration, donor companies including Eisai set a Guinness world record for “Most medication donated in 24 hours” – a total of 270 million tablets. Please click here for more details.
2021
Launch and Participation in World NTD Day
To commemorate the launch of the London Declaration, an international public-private partnership to combat NTDs, January 30th was recognized as World NTD Day by the WHO. Many events are held worldwide including a campaign to light up iconic landmarks and monuments in NTDs symbol colors (orange and purple) as well as various awareness activities to improve social attention to NTDs.
Eisai is participating in awareness activities and co-sponsoring the light up of Tokyo Tower to support WHO NTD Day related events. Please click here for more details.

(2022)

(2022)
2022
Endorsing the Public-Private Partnership “Kigali Declaration” to Combat NTDs
The Kigali Declaration on NTDs was officially launched at the Kigali Summit on Malaria and NTDs on June 23, 2022 in Kigali, Rwanda, with the aim of achieving the WHO’s NTD Roadmap 2021-2030. Serving as the successor of the London Declaration, an international public-private partnership announced in 2012, the Kigali Declaration aims to strengthen stakeholders’ commitments to NTD elimination to achieve its targets including eradicating two NTDs, eliminating at least one NTD in 100 countries and decreasing the number of people requiring interventions for NTDs by 90%. Eisai endorsed the Kigali Declaration and announced our continued support for the elimination of NTDs. Please click here for more details.

Current Distribution Status of DEC Tablets for Endemic Countries
2.68 billion tablets for 32 countries
As of September 2025
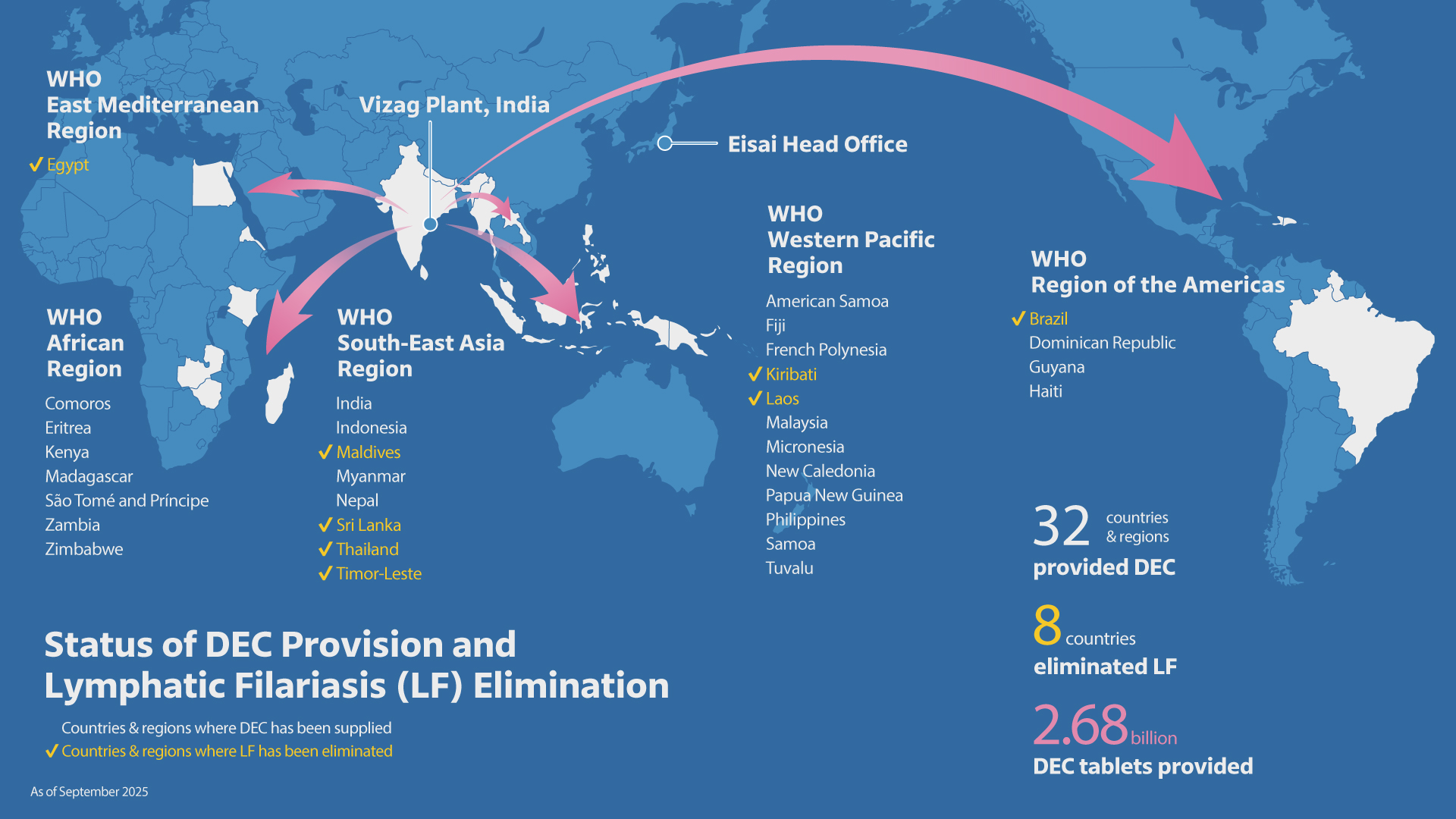
LF elimination was achieved in eight countries (Brazil, Egypt, Kiribati, Laos, Maldives, Sri Lanka, Thailand and Timor-Leste) out of 32 countries, where Eisai has provided DEC tablets.




