- For Print
- May 31, 2017
Eisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito, “Eisai”) announced today that it has entered into a new joint research agreement with the Broad Institute (Cambridge, Massachusetts, United States, “Broad”), a collaborative research institute which includes researchers from the Massachusetts Institute of Technology and Harvard University to develop a new antimalarial medicine based on antimalarial drug targets the team identified last year.
The Eisai and Broad Joint Development Program for Antimalarial Medicines, was established in September 2014 and has led to the identification and optimization of promising molecules, using hits obtained by screening Broad's compound library for antimalarials as a starting point. These compounds interact with a novel target in the malarial parasite (Phenylalanine t-RNA synthetase) which results in inhibition of protein synthesis. The compounds exhibit potent both in vitro and in vivo antimalarial activity, in the blood-, liver-, and transmission-stages of the parasite life cycle. These results were published in the scientific journal Nature in September 2016.1
Malaria is a deadly disease caused by malarial parasites and is vector-transmitted (transmitted by an infected mosquito). According to the World Health Organization, the disease led to an estimated 430,000 deaths in 2015, mostly African children.2 The majority of available antimalarial medicines target the blood-stage, in which the parasites replicate within erythrocytes. There is, however, a need for medicines that target all stages of the parasite lifecycle. Parasites can also become resistant to drug treatments, and thus there is an urgent need to develop medicines that utilize new mechanisms of action.
Under this agreement, Eisai and Broad aim to generate novel compounds with improved properties by building on the base of the joint development program's results, and leveraging Broad's medicinal chemistry capabilities alongside Eisai's knowledge of pharmaceutical development. After selection of a lead optimization candidate, Eisai will have the option to an exclusive license to develop the candidate.
“We are very encouraged by the favorable progress of our collaboration with the Broad Institute and are hopeful that it will lead to a new antimalarial medicine that will benefit the millions currently in need,” said Haruo Naito, CEO of Eisai Co., Ltd. “At Eisai, we are proactively working to contribute towards global health, which we consider to be our mission and a long term investment in creating a healthy and prosperous middle-income class.”
“If successful, this would be a novel mechanism-of-action drug that targets a protein that has never-before been targeted in antimalarial therapeutics,” said research team leader Stuart Schreiber, a founding core member of the Broad Institute and a pioneer in the field of chemical biology and novel approaches to therapeutics. “Existing drugs have been around for decades, which is one reason that resistance emerges so quickly. Eisai's commitment to working with the non-profit and academic research community to explore promising new approaches demonstrates its dedication to helping overcome a deadly medical challenge that still threatens hundreds of thousands of children in developing countries.”
This joint research program is funded by the Global Health Innovative Technology Fund (GHIT Fund), an international non-profit organization headquartered in Japan.
Under its human health care (hhc) philosophy, Eisai is determined to be proactive in improving access to medicines worldwide through partnerships with governments, international organizations, and other non-profit private sector organizations. Through these collaborations, Eisai aims to make new treatments available as early as possible to patients with malaria, tuberculosis and neglected tropical diseases, and thereby further increasing the healthcare benefits provided to the patients and their families.
<Notes to editors>
1. About the Global Health Innovative Technology Fund
The first of its kind in Japan, the GHIT Fund is a public-private partnership between the Japanese government, multiple pharmaceutical companies, the Bill & Melinda Gates Foundation, the Wellcome Trust, and UNDP. Launched in April 2013 with an initial commitment of more than US$100 million and now with capital of US$145 million, the organization taps Japanese research and development (R&D) to fight neglected diseases. GHIT Fund invests and manages a portfolio of development partnerships aimed at neglected diseases that afflict the world's poorest people. GHIT Fund mobilizes Japanese pharmaceutical companies and academic and research organizations to engage in the effort to get new medicines, vaccines, and diagnostic tools to people who need them most, with Japan quickly becoming a game-changer in global health. www.ghitfund.org.
2. About Malaria
Malaria is a deadly disease caused by malarial parasites that are transmitted to people through the bite of an infected mosquito. According to the World Health Organization, in 2015 alone, the disease infected approximately 212 million people and led to an estimated 430,000 deaths, mostly among African children.2
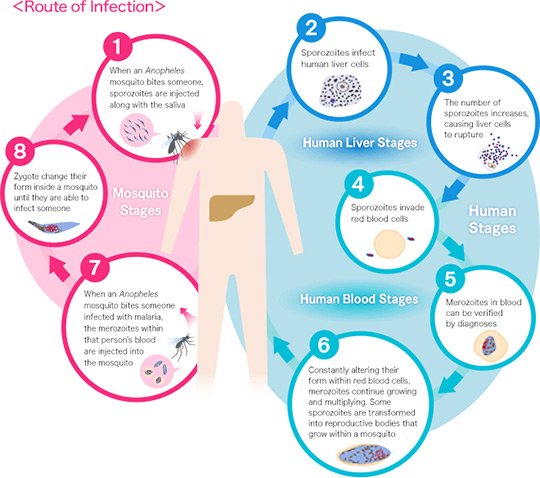
As shown in the diagram below, when a mosquito takes blood from a human, malarial parasites in sporozoite form that are injected with the saliva of the mosquito (1) grow in the person's liver cells (2 - 3: liver-stage) before then migrating to the blood stream and multiplying within red blood cells (4). This causes the red blood cells to rupture and the parasites to continue invading more red blood cells in a continuous cycle (4→5→6→4: blood-stage). The symptoms of malaria occur during this cycle, when the parasites have invaded the blood cells, and malaria treatment involves the use of medicines to work on the parasites at this stage. However, it is known that certain species of malaria such as Plasmodium vivax lie dormant in the liver cells (2) instead of growing, and after parasites in the bloodstream (4 - 6) are killed, these hidden parasites in the liver cells awaken later through some kind of stimulus and go on to reproduce and invade the bloodstream again, a process known as relapse.
In addition, most malarial parasites produced in red blood cells (merozoites) are asexual, and will die inside a mosquito if taken during feeding. However, some of these (6: transmission-stage) develop within red blood cells into male and female gametocytes that can reproduce inside mosquitoes (7 - 8), which leads to malaria transmission. As such, if these male and female gametocytes can be killed while in the bloodstream of humans, it is possible to block the transmission of malaria to mosquitoes.
3. About Malaria Treatments
The majority of available antimalarial medicines target the blood-stage, in which the parasites replicate within erythrocytes. Even though liver- and transmission-stages parasites do not cause malarial symptoms, they can lead to an onset or relapse by transitioning into the blood-stage, or they can further spread the disease by being transmitted to mosquitoes. As such, there is a need for antimalarial medicines which target all stages of the parasite lifecycle.
Furthermore, since malarial parasites develop a resistance to antimalarial medicines, there is an urgent need for antimalarial agents with new mechanisms of action. Currently, treatment for malaria combines rapidly-acting artemisinins with lumefantrine, amodiaquine, mefloquine and other antimalarials for durability. However, in recent years, there have been reports of strains of malaria having resistance to even the relatively new artemisinin medicines.
The candidate compounds discovered by Eisai and Broad have a new mechanism of action, and in pre-clinical trials, have demonstrated the rare characteristic of being effective against blood-, liver-, and transmission-stages malarial parasites. This could potentially lead to a drug that can target all stages of the parasite lifecycle.
- 1
Nobutaka Kato, et al, “Diversity-oriented synthesis yields novel multistage antimalarial inhibitors” Nature, 2016; 538, 344-349
- 2
World Health Organization http://www.who.int/mediacentre/factsheets/fs094/en/
