- For Print
- November 7, 2014
Eisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito, “Eisai”) announced today that the German Federal Joint Committee (G-BA) has determined no additional benefit for its in-house developed anti-epilepsy drug (AED) Fycompa® (perampanel) when compared to conventional AEDs in its assessment for insurance reimbursement. It is deeply regrettable that the G-BA's conclusion did not appropriately assess the clinical value brought about by the innovative properties of Fycompa or the needs of patients. Eisai will continue to seek an accurate understanding of the value of Fycompa from the G-BA.
Fycompa is a first-in-class antiepileptic drug discovered and developed by Eisai. With epileptic seizures being primarily mediated by the neurotransmitter glutamate, the agent is a highly selective, noncompetitive AMPA receptor antagonist that reduces neuronal hyperexcitation associated with seizures by targeting glutamate activity at postsynaptic AMPA receptors. Fycompa was approved for 27 countries in the EU in July 2012 and has already been launched in nine countries in the region. It is approved in more than 40 countries worldwide including the United States as an adjunctive treatment for partial-onset seizures (with or without secondary generalized seizures) in patients with epilepsy aged 12 years and older, and has been launched in 15 countries around the world.
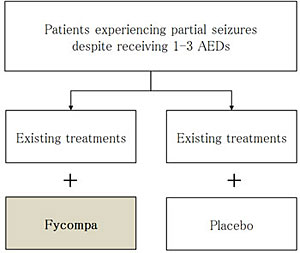
For the G-BA's assessment, Eisai submitted data from three global pivotal Phase Ⅲ studies conducted on 1,480 patients who have uncontrolled partial-onset seizures despite having been treated with other AEDs. These randomized, double-blind, placebo-controlled and dose-escalated studies showed consistent results in the efficacy and tolerability of Fycompa as an adjunctive therapy in patients with partial-onset seizures. The most commonly reported adverse events were dizziness, somnolence, fatigue, headache, falls, irritability and ataxia. A multi-center, six-month observational study from nine specialized epilepsy centers in Germany and Austria, submitted alongside the Phase Ⅲ data, demonstrates that approximately half of the 281 people with refractory epilepsy treated with Fycompa experienced at least a 50% reduction in seizure frequency and up to 15% became seizure free. The G-BA's conclusion was based not on the clinical value of Fycompa, but on a methodology that does not accept placebo-controlled study results in its assessment for insurance reimbursement instead. Since placebo-controlled study protocols for adjunctive treatment of refractory partial-onset epilepsy were accepted for the clinical studies required by the regulatory authorities in both the United States and Europe for marketing authorization, this decision is extremely unreasonable and deeply regrettable. It is highly concerning that patients in Germany will not have access to new epilepsy treatments in the future.
Epilepsy is one of the most common neurological conditions in the world and over half a million people in Germany live with the condition. The successful treatment of partial onset seizures remains a challenge; up to a third of people with epilepsy do not achieve seizure freedom despite appropriate therapy with AEDs. Patients with this difficult to treat form of epilepsy are in need of new treatment options similar to Fycompa that potentially provide greater seizure control, and these kinds of innovative medicines with new mechanisms of action are highly desired in a clinical setting.
After Fycompa was launched in Germany in September 2012, Eisai temporarily suspended distribution in Germany following the previous negative G-BA ruling in March 2013 and established a patient access program for continued supply of Fycompa free of charge to German pharmacies through individual import to ensure that people with epilepsy continue to receive treatment with Fycompa. In response to a partial revision to the additional benefit assessment system, Eisai then resubmitted Fycompa to the G-BA for additional benefit reassessment in May 2014.
Eisai remains committed to seeking out an accurate understanding and assessment from the relevant authorities regarding the value of Fycompa in order to continue to ensure delivery of the drug to patients with partial-onset seizures in Germany in need of innovative new therapies.
< Notes to editors >
1. Glossary of Terms
1) German Federal Joint Committee (G-BA)
The German Federal Joint Committee (Gemeinsamer Bundesausschuss, G-BA) is the highest decision-making body of the joint self-government of physicians, dentists, hospitals and health insurance funds in Germany. It issues directives for the benefit catalog of statutory health insurance funds (GKV) and thus specifies which drugs and medical services are reimbursed by the GKV.
2) About additional benefit assessment conducted by the G-BA
In Germany, the enactment of the Act on the Reform of the Market for Medical Products (Arzneimittelmarkt- Neuordnungsgesetz, AMNOG) came into effect on January 2011. Under this amendment, all eligible new drugs launched on the German market must undergo an additional benefit assessment conducted by the G-BA, with later price negotiations to be based on this assessment and a reimbursement price to be decided within one year from the drug's launch.
Furthermore, when a new drug is launched, the pharmaceutical company must submit to the G-BA a benefit dossier demonstrating the drug's additional benefit over a comparator. The G-BA then usually commissions the country's Institute for Quality and Efficiency in Health Care (IQWiG) to evaluate the dossier to decide whether any additional benefit exists over the comparator and the results are subsequently published. The pharmaceutical company is next given an opportunity to comment at a hearing on the IQWiG's evaluation, after which the G-BA carries out its final decision regarding any additional benefit of the drug.
If an additional benefit is recognized by the G-BA, the drug proceeds to the price negotiation stage with the lead association of the German sick funds (GKV-SV), and a reimbursement price is decided based on the level of additional benefit as decided by the G-BA. On the other hand, a drug deemed to offer no recognized additional benefit is designated a reference price group as well as a reimbursement price based on the price of the least expensive existing drug, including generics.
2. About Adjunctive Treatment of Epilepsy
The goal of treating patients with epilepsy is seizures-free. Once epilepsy is diagnosed, a patient usually begins monotherapy (single-agent) treatment with an antiepileptic drug. In cases where the treatment's efficacy on seizure control is inadequate, the initial treatment can typically be replaced with a different monotherapy. In approximately 30-40% of patients, however, seizure control is not achieved even after undergoing two different monotherapy treatments and in these circumstances the patient can then opt to switch to a treatment that includes an adjunctive therapy. In these cases, adjunctive therapies like Fycompa are used as an “add-on” to the patient's existing treatment. Based on these types of clinical conditions, it has become the standard for placebo to be used as the comparator “add-on” to existing treatments in clinical studies in guidelines on clinical development for the pharmaceutical approval of new refractory epilepsy treatments. Phase Ⅲ clinical studies of Fycompa were conducted in accordance with such guidelines.
3. About Fycompa
Fycompa, a novel chemical entity discovered and developed by Eisai, is a noncompetitive AMPA-type glutamate receptor antagonist. Fycompa is an antiepileptic drug that reduces neuronal hyperexcitation associated with seizures by targeting glutamate activity at postsynaptic AMPA receptors. The agent is currently approved in more than 40 countries and territories, including Europe and the United States, as an adjunctive treatment (once-daily oral dose) of partial-onset seizures and is also being evaluated in a Phase Ⅲ study (Study 335) in Asia, including Japan.
A Phase Ⅲ study (Study 332) of the agent as an adjunctive therapy for the treatment of primary generalized tonic-clonic seizures (PGTC) conducted in the United States, Europe and Asia, including Japan, met its primary endpoint, and the regulatory applications for an indication expansion of the agent are under review in the United States and Europe. The company plans to submit a regulatory application covering both study 332 and study 335 in Japan in fiscal 2015. Furthermore, Eisai is conducting Phase Ⅱ studies in Europe and the United States for partial-onset epilepsy in pediatric patients.
