- For Print
- May 12, 2014
Eisai Co., Ltd. (Headquarters: Tokyo, President & CEO: Haruo Naito, “Eisai”) announced today that its German sales company Eisai GmbH (Location: Frankfurt) has resubmitted its first-in-class anti-epilepsy drug (AED) Fycompa® (AMPA receptor antagonist, generic name: perampanel) to the German Federal Joint Committee (G-BA) for additional benefit assessment. The new decision is expected to be published within 6 months.
In Germany approximately 3,000 and 4,000 people with epilepsy have been treated with Fycompa since its launch in September 2012. Eisai temporarily suspended Fycompa from distribution in Germany following the previous negative G-BA ruling in March 2013 and established a patient access program for continued supply of Fycompa free of charge to German pharmacies through individual import to ensure that people with epilepsy continue to receive treatment with Fycompa, while the G-BA considers the resubmission.
In Germany, approximately one in every 200 people has epilepsy, which equates to an estimated 400,000 people in the country who live with the condition. The successful treatment of partial-onset seizures remains a challenge as over 30% of patients do not achieve adequate seizure control despite appropriate therapy with existing AEDs, and there continues to be a pressing need for innovative new treatments. Fycompa was approved in over 35 countries in Europe, North America and Asia as an adjunctive treatment for partial-onset seizures (including secondarily generalized seizures) in patients with epilepsy aged 12 years and older.
Eisai believes that through this reassessment of additional benefit, Fycompa will be recognized appropriately for its benefits as well as being an innovative new treatment, and that sales of the drug will be resumed in Germany, ensuring delivery to patients with partial-onset seizures.
[ Please refer to the following notes for further information on Fycompa and a glossary of terms. ]
< Notes to editors >
1. About Fycompa® (Perampanel)
Fycompa (perampanel), a novel chemical entity discovered and developed by Eisai, is a noncompetitive AMPA-type glutamate receptor antagonist. Fycompa is the first AED to reduce neuronal hyperexcitation associated with seizures by targeting glutamate activity at postsynaptic AMPA receptors and has demonstrated its antiseizure effects in Phase Ⅱ and Ⅲ studies. The agent is currently approved in more than 35 countries and territories, including in Europe and the United States, as a treatment (once-daily oral dose) of partial-onset seizures and is also being evaluated in a Phase Ⅲ study in Asia, including in Japan. Furthermore, Eisai is conducting a global Phase Ⅲ perampanel study for generalized epilepsy and Phase Ⅱ perampanel studies in Europe and the United States for partial-onset epilepsy in pediatric patients, as it seeks to expand the range of indications for which the drug is approved.
2. Glossary of Terms
1) German Federal Joint Committee (G-BA)
The German Federal Joint Committee (Gemeinsamer Bundesausschuss, G-BA) is the highest decision-making body of the joint self-government of physicians, dentists, hospitals and health insurance funds in Germany. It issues directives for the benefit catalog of statutory health insurance funds (GKV) and thus specifies which drugs and medical services are reimbursed by the GKV.
2) About additional benefit assessment conducted by the G-BA
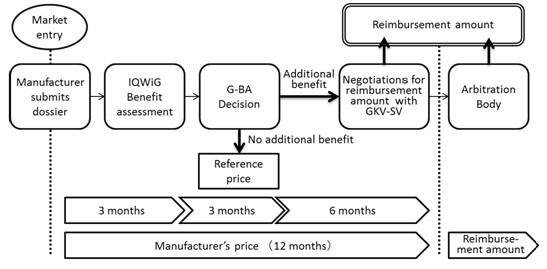
In Germany, the enactment of the Act on the Reform of the Market for Medical Products (Arzneimittelmarkt-Neuordnungsgesetz, AMNOG) came into effect on January 2011. Under this amendment, all eligible new drugs launched on the German market must undergo an additional benefit assessment conducted by the G-BA, with later price negotiations to be based on this assessment and a reimbursement price to be decided within one year from the drug's launch.
Furthermore, when a new drug is launched, the pharmaceutical company must submit to the G-BA a benefit dossier demonstrating the drug's additional benefit over a comparator. The G-BA then usually commissions the country's Institute for Quality and Efficiency in Health Care (IQWiG) to evaluate the dossier to decide whether any additional benefit exists over the comparator and the results are subsequently published. The pharmaceutical company is next given an opportunity to comment at a hearing on the IQWiG's evaluation, after which the G-BA carries out its final decision regarding any additional benefit of the drug.
If an additional benefit is recognized by the G-BA, the drug proceeds to the price negotiation stage with the lead association of the German sick funds (GKV-SV), and a reimbursement price is decided based on the level of additional benefit as decided by the G-BA. On the other hand, a drug deemed to offer no recognized additional benefit is designated a reference price group as well as a reimbursement price based on the price of the comparator used during the benefit assessment.