- For Print
- July 29, 2013
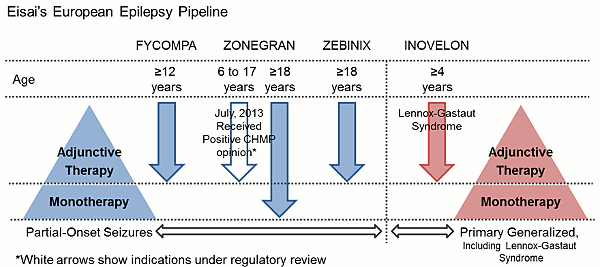
Eisai Co., Ltd. (Headquarters: Tokyo, President & CEO: Haruo Naito, “Eisai”) announced today that it has received a positive opinion from the European Medicines Agency (EMA)'s Committee for Medicinal Products for Human Use (CHMP) on the license extension application submitted by its U.K. subsidiary Eisai Ltd. regarding the use of antiepileptic agent Zonegran® (zonisamide) in the treatment of pediatric patients. The application seeks to extend the use of adjunctive Zonegran in the treatment of partial seizures (with or without secondary generalization) by changing the approved age range from adults aged 18 years and above to also include pediatric patients aged 6 years and above.
Zonegran is an antiepileptic drug (AED) originally created by Dainippon Pharmaceutical Co., Ltd. (currently Dainippon Sumitomo Pharma Co., Ltd.), for which Eisai has been pursuing development of the agent in Europe. Zonegran was first approved in March 2005 as an adjunctive therapy for the treatment of partial-onset seizures (with or without secondary generalization) in adults with epilepsy and received additional approval on June 27, 2012, as a monotherapy for partial-onset seizures in adults with newly diagnosed epilepsy. The agent is currently marketed in Europe by Eisai's subsidiaries.
In a double-blind, randomized, multicenter, placebo-controlled, pivotal Phase III study (Study 312) conducted to evaluate adjunctive zonisamide in 207 pediatric patients (6 to 17 years) with partial seizures who had received one or two AEDs, results showed that the proportion of responders (defined as 50% or greater reduction in seizure frequency) was significantly higher with zonisamide versus treatment with placebo. The overall incidence of treatment-emergent adverse events (TEAEs) was similar for zonisamide versus placebo. TEAEs reported more frequently with zonisamide versus placebo were decreased appetite, decreased weight, somnolence, vomiting and diarrhea.
Eisai defines epilepsy as a therapeutic area of focus and as part of its European epilepsy pipeline markets the novel, in-house-discovered AMPA receptor antagonist Fycompa® (perampanel), Zonegran® and Zebinix® as treatments for partial seizures and Inovelon® as a treatment for seizures associated with Lennox-Gastaut syndrome, a severe form of childhood-onset epilepsy. By offering multiple treatment options as part of its abundant product portfolio in the epilepsy area, Eisai seeks to make further contributions to address the diversified needs of, and increase the benefits provided to, patients with epilepsy and their families.
[ Please refer to the following notes for further information on Zonegran Study 312 and Eisai's Commitment to Epilepsy. ]
Media Inquiries:
Public Relations Department,
Eisai Co., Ltd.
+81-(0)3-3817-5120
< Notes to editors >
1. About Zonegran® (zonisamide) Study 312
Study 312 was a double-blind, randomized, multicenter, placebo-controlled study conducted in the European Union and India to evaluate adjunctive zonisamide in 207 pediatric patients (6 to 17 years) with partial seizures who had received one or two antiepileptic drugs. Patients were assigned to receive either placebo or zonisamide for 20 weeks (8 weeks of titration, 12 weeks of maintenance therapy). The percentage of patients who completed the study was comparable between the zonisamide and placebo groups (86.9% of patients given zonisamide and 90% of patients given placebo). Results showed that the proportion of responders (defined as 50% or greater reduction in seizure frequency) after the 12-week maintenance treatment, the study's primary endpoint, was significantly higher with zonisamide (50.5%) versus treatment with placebo (31.0%). Safety and tolerability assessments showed that the overall incidence of TEAEs was similar for zonisamide (55.1%) versus placebo (50.0%). There were low rates of serious TEAEs in the zonisamide and placebo groups (3.7% versus 2.0%), and TEAEs leading to withdrawal from the study (0.9% versus 3.0%). TEAEs reported more frequently with zonisamide versus placebo wer decreased appetite (6.5% versus 4.0%), decreased weight (4.7% versus 3.0%), somnolence (4.7% versus 2.0%), vomiting (3.7% versus 2.0%) and diarrhea (3.7% versus 1.0%).
2. Eisai's Commitment to Epilepsy
Eisai defines epilepsy as a therapeutic area of focus. As of July 2013, the company's in-house-developed AMPA receptor antagonist Fycompa® (perampanel) has been approved as a novel treatment for partial seizures in over 30 countries, including the United States and in Europe, having been launched in various EU member states since September 2012. As a part of its European epilepsy pipeline, the company currently also markets Zonegran® (under license from Dainippon Sumitomo Pharma Co., Ltd.; sodium/calcium channel-blocking antiepileptic agent) and Zebinix® (under license from BIAL-Portela & Ca S.A.; voltage-dependent sodium channel-blocking antiepileptic agent) as adjunctive therapies in adults with partial seizures as well as Inovelon® (under license from Novartis AG; sodium channel-blocking novel triazole-derived antiepileptic agent) for adjunctive treatment of seizures associated with Lennox-Gastaut syndrome, a severe form of early childhood-onset epilepsy. In June 2012, Zonegran was approved in the EU for the additional indication of monotherapy for the treatment of partial seizures in adults with newly diagnosed epilepsy, and Eisai has now also received a positive opinion from the European Medicines Agency (EMA)'s Committee for Medicinal Products for Human Use (CHMP) regarding its application to extend the adjunctive use of the agent in the treatment of partial seizures to include pediatric patients.