- For Print
- September 11, 2012
Roche Diagnostics K.K.
Eisai Co., Ltd.
EIDIA Co., Ltd.
Roche Diagnostics K.K. (Headquarters: Tokyo, President and CEO: Makoto Ogasawara, “Roche Diagnostics”) together with Eisai Co., Ltd. (Headquarters: Tokyo, President and CEO: Haruo Naito, “Eisai”) and its diagnostics subsidiary EIDIA Co., Ltd. (Headquarters: Tokyo, President: Atsushi Saito, “EIDIA”) announced today that CoaguChek® XS Personal (specially controlled medical device/medical equipment requiring specialist maintenance and management), a coagulation analyzer for patient self-testing approved in Japan on July 27, 2012, has received reimbursement approval from Japan’s Ministry of Health, Labor and Welfare for use in the self-testing of patients fitted with a Nonpulsatile Left Ventricular Assist System (LVAS) and will be launched in Japan on September 25.
The three companies have been collaborating on the distribution and promotion of the CoaguChek XS Series of coagulation monitoring devices for use by healthcare professionals since 2008. As with the existing products in this series, CoaguChek XS Personal will be manufactured and distributed by Roche Diagnostics, with distribution and co-promotion being undertaken by EIDIA and Eisai, respectively.
Patients fitted with a Nonpulsatile LVAS are at a higher risk of cardiogenic cerebral embolisms and require anticoagulation therapy. Warfarin (warfarin potassium), an oral anticoagulant currently manufactured and marketed by Eisai, is recommended as a firstline therapy for the treatment and prevention of cardiogenic cerebral embolisms, however, in order to ensure the safe use of the drug, patients on Warfarin must have their blood-clotting ability carefully monitored through measurement of PT/INR (Prothrombin Time/International Normalized Ratio), a measure of blood-clotting ability.
CoaguChek XS Personal is a small, easy-to-use self-testing coagulation analyzer that patients taking Warfarin can use, under the guidance and supervision of a physician, to measure their PT/INR in approximately one minute in order to monitor their blood-clotting ability without the need to visit a medical facility for testing. The device is restricted for use only by those patients who have been fitted with a Nonpulsatile LVAS or mechanical heart valve. CoaguChek XS Personal received reimbursement approval on September 7, 2012 for use as an at-home self-testing device by patients fitted with a Nonpulsatile LVAS, enabling all fees associated with use of the device to be processed as a medical treatment fee-“C116 Supervision Fee for At-home Testing Associated with Nonpulsatile LVAS.” It is expected that coverage of the device under Japan’s National Health Insurance (NHI) system will help not only reduce the financial burden on patients, but also to improve their quality of life. Currently, patients fitted with a mechanical heart valve are not covered under NHI for at-home hemostatic testing using this device.
Going forward, Roche Diagnostics, Eisai and EIDIA will continue to make further contributions to ensure the appropriate management of Nonpulsatile LVAS patients on oral anticoagulation therapy and to improve their QOL.
[ Please refer to the following notes for a glossary of terms, product outline and corporate profiles ]
[ Contacts: ]
< Media Inquiries >
Corporate Planning and Communications,
Roche Diagnostics K.K.
Tel: +81-(0)3-5443-7040
Public Relations Department,
Eisai Co., Ltd.
Tel: +81-(0)3-3817-5120
Public Relations Section,
EIDIA Co., Ltd.
Tel: +81-(0)3-3865-4311
< Product Inquiries >
Customer Support Center,
EIDIA CO., Ltd.
Tel. 0120-921-207
< Notes to Editors >
[ Glossary of Terms ]
- 1)
Prothrombin
Prothrombin is a blood coagulation factor. Blood coagulation time gets longer when the amount of prothrombin in the blood decreases due to deterioration of liver function caused by hepatic diseases or other conditions.
- 2)
PT (Prothrombin Time)
PT is measured to check how long it takes blood to clot. A PT test can be used to screen for bleeding problems and assess liver damage as well as to check to see whether or not oral anticoagulants such as warfarin are working.
- 3)
PT/INT (Prothrombin Time/International Normalized Ratio)
As the Quick/PT value is very country- and laboratory- dependent, nowadays the value mostly measured is the INR value. It is the globally recommended unit for measuring thromboplastin time. INR makes coagulation measurements extensively comparable despite the numbers of different thromboplastins used.
- 4)
Cardiogenic Cerebral Embolism
A type of stroke that occurs when blood clots formed in the heart travel to and block an artery that supplies the brain. This kind of embolism occurs predominantly in people with cardiac diseases such as arrhythmia (in particular atrial fibrillation) or heart valve disease.
[Product Outline]
1.Specifications
*You can scroll to the left or right here
| External Dimensions | 78mm(W) x 138mm(D) x 28mm (H) |
| Measurement Range | INR: 0.8~8.0 |
| Measurement Time | Approximately 1 minute |
| Weight | 127g (excluding batteries) |
| Sample Type Measured | Capillary blood or non-anticoagulated whole blood |
| Sample Size | 10μL or more |
2.Manufacturer’s Suggested Retail Price
*You can scroll to the left or right here
| 1) | CoaguChek® XS Personal Starter Kit | ¥121,000 |
| Main Unit | 1 meter | |
| SafetyPro Plus (Fingerstick) | 25 sticks | |
| Note) Reagent is sold separately. | ||
| 2) | CoaguChek®XS Personal (unit only) | ¥120,000 |
| Note) Reagent is sold separately |
3.Manufacture and Distribution
*You can scroll to the left or right here
| Manufactured and distributed by: | Roche Diagnostics K.K. |
| Distributed by: | EIDIA Co., Ltd. |
| Co-promoted by: | Eisai Co., Ltd. |
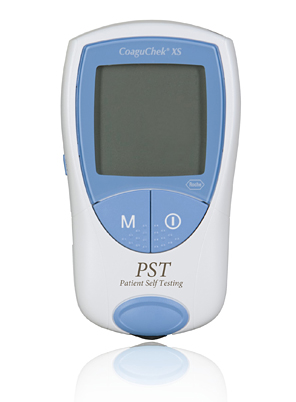
4.Product Photograph
< This photo is available for download from the Roche Diagnostics K.K. corporate website >
http://www.roche-diagnostics.jp/news/photo/index.html
[ Corporate Profiles ]
■Roche Diagnostics K.K.
*You can scroll to the left or right here
| Headquarters | 2-6-1 Shiba, Minato-ku, Tokyo, Japan |
| Representative | Makoto Ogasawara, President & CEO |
| Capital | 2,500 million yen (as of December 31, 2011) |
| Operations | Manufacturing, marketing and import of in vitro diagnostics, laboratory instruments and raw materials for diagnostics/pharmaceuticals. |
| Corporate Website | http://www.roche-diagnostics.jp/ |
■Eisai Co., Ltd.
*You can scroll to the left or right here
| Headquarters | 4-6-10 Koishikawa, Bunkyo-ku, Tokyo, Japan |
| Representative | Haruo Naito, President & CEO |
| Capital | 44,985 million yen (as of March 31, 2012) |
| Operations | Research and development, manufacturing, marketing and import/export of pharmaceuticals. |
| Corporate Website | http://www.eisai.com |
■EIDIA Co., Ltd.
*You can scroll to the left or right here
| Location of headquarters | 1-10-6 Iwamoto-cho, Chiyoda-ku, Tokyo, Japan |
| Representative | Atsushi Saito, President |
| Capital | 5,262 million yen (as of March 31, 2012) |
| Operations | Research and development, manufacturing, marketing and import/export of in vitro diagnostics, laboratory reagents, medical equipment and other supplies. |
| Corporate Website | http://www.eidia.co.jp/en/index.html |
