- For Print
- July 19, 2012
Eisai Co., Ltd. (Headquarters: Tokyo, President & CEO: Haruo Naito, “Eisai”) announced today that the first data from clinical studies with E2609, a BACE (beta-site amyloid precursor protein-cleaving enzyme) inhibitor discovered and being developed in-house as a treatment for Alzheimer’s disease, were presented during oral sessions at the Alzheimer’s Association International Conference (AAIC) 2012.
β-amyloid (Aβ) deposition in the brain is thought to be one of causes of Alzheimer’s disease, and it is expected to develop a new treatment for Alzheimer’s disease with reducing Aβ which not only improves symptoms, but also has modulatory effects such as slowing down the progression of the disease. E2609 reduces the overall amount of Aβ by inhibiting BACE. The two E2609 Phase I studies presented at the AAIC 2012 comprised a single oral ascending dose study (Study A001-001) which showed Aβ levels reduction in plasma, and a 14-day multiple oral ascending dose study (Study A001-002) which showed dose-dependent increase of E2609 PK level and statistically significant reduction of Aβ levels in cerebrospinal fluid (CSF). This Phase I study results confirmed the “Proof of Mechanism” for E2609 of inhibiting Aβ production by BACE inhibition in human.
Study A001-001 was a randomized, double-blind, placebo-controlled, single ascending dose study conducted in 73 healthy adult volunteers. Subjects were divided into nine cohorts to receive E2609 doses of between 5 mg to 800 mg. Plasma Aβ [Aβ(1-x)] levels were measured prior to patients receiving E2609 and then at multiple time points up to 144 hours post-dose. The maximum dose-dependent reduction of plasma Aβ(1-X) relative to baseline was 52% at 5 mg, and 92% at 800 mg. E2609 showed acceptable tolerability across all doses, and the most common adverse events included headache and dizziness.
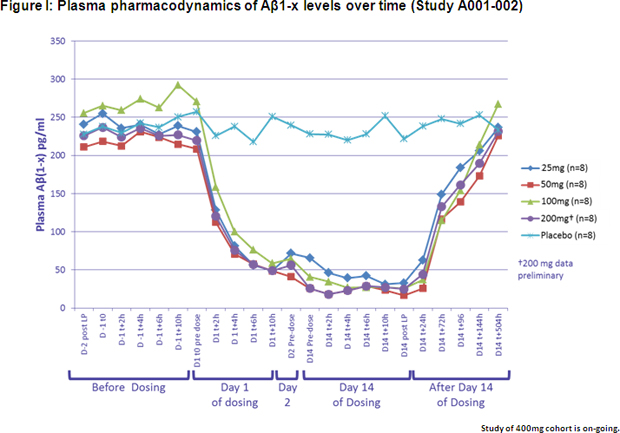
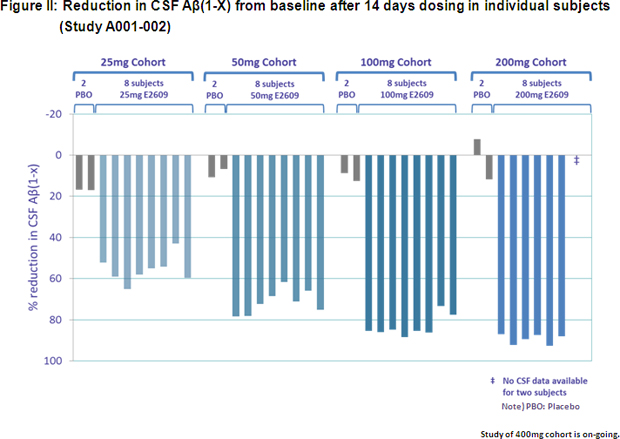
Study A001-002 was a randomized, double-blind, placebo-controlled, multiple ascending dose study conducted in 50 healthy adult volunteers. Subjects were divided into five cohorts, with each cohort receiving E2609 doses of between 25 mg and 400 mg per day for 14 days. The study measured Aβ levels both in plasma and CSF. Preliminary interim analysis results showed that E2609 demonstrated a clear reduction in plasma Aβ(1-X) level (See Figure I) and a dose-dependent reduction in CSF Aβ(1-X) (See Figure II) in subjects who received daily doses of between 25 mg and 200 mg. The percentage decrease in CSF Aβ(1-x) after 14 days dosing compared to baseline was statistically significant, with E2609 demonstrating a 46.2%, 61.9%, 73.8% and 79.9% difference in percentage decrease compared to placebo in the 25 mg, 50 mg, 100 mg and 200 mg cohorts, respectively. No clinically significant safety concerns were observed following repeated oral dosing of up to 200 mg. Several cases of orolabial herpes relapse were observed in the 200 mg cohort, however, while being evaluated, the significance of these cases is unknown as the blind remains unbroken on the safety data.
Since launching the Alzheimer’s disease treatment Aricept®, Eisai has been committed to enhancing the value that the drug provides patients through the development of new formulation types and new indications, the carrying out of disease awareness-raising activities aimed at promoting early diagnosis and treatment, and the improvement of diagnostic technologies. However, despite these efforts, Alzheimer’s disease still remains an area with a large number of unmet medical needs. Aiming to develop next-generation Alzheimer’s disease treatments, Eisai is also pursuing the development of the novel monoclonal antibody BAN2401 targeting for Aβ protofibrils and other compounds in addition to E2609 as it seeks to make further contributions to address the diversified needs of, and increase the benefits provided to, Alzheimer’s disease patients and their families.
Media Inquiries:
Public Relations Department,
Eisai Co., Ltd.
+81-(0)3-3817-5120