- For Print
- October 27, 2010
Pfizer Japan Inc.
Eisai Co., Ltd.
Tokyo, Japan, October 27, 2010- Today, October 27, 2010, Pfizer Japan Inc. (Head Office: Tokyo; President: Ichiro Umeda; hereinafter “Pfizer”) received approval to replace the indication of “postherpetic neuralgia” that is currently approved for Lyrica® Capsules (generic name: pregabalin; hereinafter “Lyrica®”) in Japan with the new and broader indication of “peripheral neuropathic pain.”
Approval to produce and distribute Lyrica with the indication postherpetic neuralgia was received on April 16 of this year, and it was launched on June 22. Pfizer and Eisai Co., Ltd. (Head Office: Tokyo; President: Haruo Naito) are jointly promoting the drug in Japan and providing information regarding the proper usage of the drug.
Lyrica® is a therapeutic agent for the treatment of pain developed by Pfizer Inc. (USA). It has been approved in 110 countries and regions worldwide (as of July 2010). The drug is recommended by the International Association for the Study of Pain and other key academic bodies as a first-line treatment for neuropathic pain. As its major mechanism of action, Lyrica® is thought to express its analgesic effect by inhibiting the release of various neurotransmitters in an overexcited nervous system.
The pathologies and pathogeneses of neuropathic pain are complex and varied, so it is considered to be a form of intractable pain for which NSAIDs (non-steroidal anti-inflammatory drugs) and other analgesics cannot be expected to have much effect.
Lyrica® has a mechanism of action that differs from those of conventional analgesic agents, so it is a new option for the treatment of pain. Its efficacy and safety have been confirmed in domestic Phase III trials and domestic long-term administration trials regarding pain accompanying diabetic neuropathy in addition to postherpetic neuralgia, a typical peripheral neuropathic disease for which it was already approved.
Pfizer Japan Inc. and Eisai Co., Ltd. will use the newly approved indication of peripheral neuropathic pain for Lyrica® to help improve the quality of life of patients suffering from pain accompanying various types of neuropathic disorders.
Press contact
-
Pfizer Japan Inc.
Product Communications
-
Eisai Co., Ltd.
Public Relations Department
Contact for healthcare professionals and consumers regarding Lyrica® (toll-free in Japan)
-
Pfizer Japan Inc.
Product Information Center
-
Eisai Co., Ltd.
Customer Hotline
Outline of Lyrica® Capsules
*You can scroll to the left or right here
| Product name: | Lyrica® Capsules (25mg, 75mg, 150 mg) |
|---|---|
| Generic name: | Pregabalin |
| Approval date: | April 16, 2010 |
| NHI pricing date: | June 11, 2010 |
| Launch date: | June 22, 2010 |
| Manufactured/sold by: | Pfizer Japan Inc. |
| Co-promotion with: | Eisai Co., Ltd. |
| Effect/efficacy: | Peripheral neuropathic pain (NOTE: changed from postherpetic neuralgia) |
| Administration/dosage: | Normally, adults are orally administered an initial dosage of 150mg of pregabalin in two divided doses per day. The daily dosage is then titrated to 300mg over one week. The dosage may be adjusted according to age and symptoms. However, daily dosage should never exceed 600mg and should always be orally administered in two divided doses per day. |
| Properties: |
|
< Notes >
Regarding pain classifications
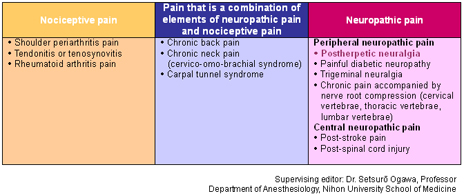
Pain is classified into three categories according to its mechanism and characteristics: neuropathic pain, nociceptive pain, and psychogenic pain. It is thought that these categories often overlap rather than existing independently.*
Neuropathic pain has been defined by the International Association for the Study of Pain (IASP) as “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system.” In other words, it is pain that occurs as a result of nerve damage or a functional anomaly accompanying such damage, and it indicates a variety of pain types accompanied by disturbance of perception.
In addition, neuropathic pain can be categorized as peripheral or central according to the site of the nerve lesion. Typical peripheral neuropathic pain disorders include postherpetic neuralgia, painful diabetic neuropathy, and trigeminal neuralgia, whereas post-stroke pain is a central neuropathic pain disorder.
Nociceptive pain is pain that occurs when a pain-producing substance that is activated by a nociceptive stimulus or inflammation stimulates nociceptors. It has been defined by the IASP as pain arising from activation of nociceptors.
Nociceptive pain includes shoulder periarthritis pain and rheumatoid arthritis pain.
Some pain is a combination of neuropathic pain and nociceptive pain. Chronic back pain and cervico-omo-brachial syndrome are representative examples of this.
The World Health Organization classifies psychogenic pain as a somatoform disorder in the International Classification of Diseases, and it is incorporated into the entry “Pain Disorder” in the DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition) of the American Psychiatric Association.
Conceptually speaking, pain is considered to be psychogenic (1) when there is no organic lesion and psychological factors account for all of the causes of the pain, or (2) when an organic physical lesion exists as a source of the pain, but it does not sufficiently explain the pain complaint.**