- For Print
- December 4, 2007
Eisai Co., Ltd. (Headquarters: Tokyo, President and CEO: Haruo Naito) and BioArctic Neuroscience AB (Headquarter: Stockholm, Sweden, CEO: Pär Gellerfors) announced today that on December 3, they signed an exclusive licence agreement for BAN2401, a novel humanized monoclonal antibody, which is being developed as a next-generation therapeutic treatment for Alzheimer's disease. Under the terms of the agreement Eisai obtains the global rights to study, develop, manufacture and market BAN2401 for the treatment of Alzheimer's disease.
BAN2401 is the result of a strategic research alliance between Eisai and BioArctic initiated in 2005 to identify a potential immunotherapy for Alzheimer's disease. The research is based on the Arctic mutation of amyloid beta-peptide (Aβ), discovered by Prof. Lannfelt at Uppsala University, which causes familial Alzheimer's disease.
BAN2401 is a humanized monoclonal antibody which selectively recognizes Aβ protofibrils, a form of soluble aggregate of Aβ believed to play a key role in the development of Alzheimer's disease. The antibody is currently in pre-clinical development and Eisai aims to develop a novel treatment for Alzheimer's disease using this antibody.
The licensing agreement for BAN2401, together with the gamma secretase modulator E2012 developed in-house by Eisai, allows the Company to pursue parallel approaches to developing next-generation treatments for Alzheimer's disease based on a small molecule compound and immunotherapy.
As a pioneer in Alzheimer's disease treatment, having discovered and developed Aricept®(donepezil hydrochloride), Eisai aims to accelerate the development of a new generation of treatments for Alzheimer's disease through in-house activities and alliances with outside organizations.
[Please see the following notes for corporate profiles, descriptions for specific terms, Eisai's commitment to developing new therapy for Alzheimer's disease]
< Note to Editors >
1. BioArctic Neuroscience AB
BioArctic Neuroscience is a biopharmaceutical company established in 2003 with the aim of commercializing the research in Alzheimer's disease carried out by the internationally renowned researcher Prof. Lannfelt at Uppsala University.
2. Pathogenesis of Alzheimer's disease and Aβ protofibrils
Accumulation of Aβ in the brain is believed to play a key role in the development of Alzheimer's disease. Pathologically, the accumulation is found as senile plaques containing aggregated insoluble Aβ fibrils.
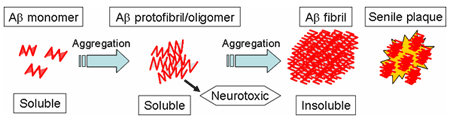
It is described that the toxic species causing Alzheimer's disease are the precursor forms of insoluble Aβ fibrils, the soluble Aβ aggregates including Aβ protofibrils.
3. Immunotherapy for Alzheimer's disease
As immunotherapies for Alzheimer's disease, there exist vaccine therapies that use Aβ to induce anti-Aβ immunoresponses, and antibody therapies that administrate monoclonal antibodies targeting Aβ. BAN2401 targets brain Aβ protofibrils by applying BioArctic Neuroscience's technology and it can efficiently eliminate Aβ species causing Alzheimer's disease.
4. Arctic mutation in Aβ
The Arctic mutation, which is one amino acid substitution in the Aβ peptide was discovered by Prof. Lannfelt at Uppsala University. This mutation causes familial Alzheimer's disease.
5. Eisai's commitment to providing new therapy for Alzheimer's Disease
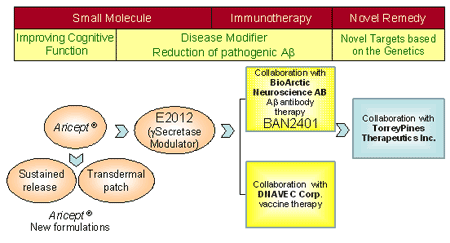
E2012 and BAN2401
E2012 is a small molecule gamma secretase modulator developed in-house by Eisai, which inhibits Aβ production. Meanwhile, BAN2401 is a humanized monoclonal antibody for immunotherapy, which eliminates Aβ species believed to play a key role in the development of Alzheimer's disease.
