- For Print
- July 11, 2007
Eisai Co., Ltd. (Headquarters: Tokyo, President and CEO: Haruo Naito) announced the launch of “Nitorol® Injection 5 mg Syringe” and “Nitorol® Continuous Intravenous Infusion 25 mg Syringe” (generic name: isosorbide dinitrate), the first nitric acid syringe formulations approved for the treatment of acute cardiac failure and unstable angina in Japan. The new formulations will be available from July 12, 2007.
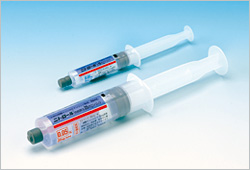
“Nitorol® Injection 5 mg Syringe” and “Nitorol® Continuous Intravenous Infusion 25 mg Syringe” are pre-filled syringes that contain the same solution as Eisai's existing ampoule formulation of “Nitorol® Injection 5 mg” (0.05% solution of isosorbide dinitrate). Unlike ampoules that require transferring the solution to syringes, the new formulations can be used with simple and easy preparation. Pre-filled syringes can also prevent foreign contamination of substances from getting into the solution.
“Nitorol®” has been contributing to the treatment of ischemic heart disease. By dilating arteries and veins, it reduces cardiac work loads. It also dilates coronary arteries and thereby improves the ischemic conditions. In addition, the rapid onset of action of “Nitorol® Injection” has demonstrated its utility in the treatment of acute heart failure and unstable angina, where fast treatment and reliable efficacy are vital.
Of the two newly introduced formulations, “Nitorol® Injection 5 mg Syringe” enables a shorter preparation process that is necessary in such occasions where a prompt injection of nitric acid is required in the catheter room. “Nitorol® Continuous Intravenous Infusion 25 mg Syringe” is designed to fit to precision programmable syringe pumps, supporting an early initiation of medical treatment.
Since the launch of “Nitorol® Injection 5 mg” in Japan in June 1986, Eisai has expanded the “Nitorol® Injection” product line by adding vial and soft bag formulations. The introduction of pre-filled syringe formulations will strengthen Eisai's commitment to make further contributions to improve usability and safety management in acute cardiac failure and unstable angina treatments.
[Please see the following notes for the product information and the product image]
< Notes to Editor >
About Nitorol® Injection 5 mg Syringe
- Active Ingredient (Generic Name): isosorbide dinitrate
- Indications:
Acute heart failure (including acute exacerbation stage of chronic heart failure)
Unstable angina pectoris
Remission of vasospasms in coronary angiography
- Dosage and Administration :
Acute heart failure
In usual adults, Nitorol® is prepared as an undiluted solution or a 0.05-0.001% solution diluted with isotonic sodium chloride solution, 5% glucose injection or other diluent, and drip-infused intravenously at a dose of 1.5-8 mg/hr of isosorbide dinitrate. The dosage of drip infusion should be adjusted depending on the patient's condition, and if necessary, may be increased up to 10 mg/hr.Unstable angina pectoris
In usual adults, Nitorol® is prepared as an undiluted solution or a 0.05-0.001% solution diluted with isotonic sodium chloride solution, 5% glucose injection or other diluent, and drip-infused intravenously at a dose of 2-5 mg/hr of isosorbide dinitrate. The dosage of drip infusion should be adjusted depending on the patient's condition.Remission of vasospasms in coronary angiography
In usual adults having coronary angiography, Nitorol® is catheterized at a dose of 5 mg of isosorbide dinitrate as undiluted solution, and infused via Valsalva's sinus within 1 min. The dosage should be adjusted depending on the patient's condition, and if necessary, may be increased up to 10 mg.<Precaution>
If Nitorol® induces vasospasms during coronary angiography, measures for these remissions should be taken immediately. Dangerous arrhythmias such as ventricular fibrillation or decrease in blood pressure due to reperfusion injury during remission of complete coronary obstruction may occur rarely. In the event of such symptoms, appropriate measures, such as electrical defibrillation, should be taken.
About Nitorol® Continuous Intravenous Infusion 25 mg Syringe
Active Ingredient (Generic Name): isosorbide dinitrate
Indications:
Acute heart failure (including acute exacerbation stage of chronic heart failure)
Unstable angina pectoris
Dosage and Administration :
Acute heart failure
In usual adults, Nitorol® is continuously infused intravenously at a dose of 1.5-8 mg/hr of isosorbide dinitrate. The dosage of drip infusion should be adjusted depending on the patient's condition, and if necessary, may be increased up to 10 mg/hr.Unstable angina pectoris
In usual adults, Nitorol® is continuously infused intravenously at a dose of 2-5 mg/hr of isosorbide dinitrate. The dosage of drip infusion should be adjusted depending on the patient's condition.
Date of Approval
March 15, 2007
NHI Price (per syringe) *Listed on June 15, 2007
Nitorol® Injection 5 mg Syringe: 433 yen
Nitorol® Continuous Intravenous Infusion 25 mg Syringe: 1,351 yen
Date of Launch
July 12, 2007
Other Products in the Nitorol® Injection Product Line
*You can scroll to the left or right here
| Product Name | Formulation | Date of Approval |
|---|---|---|
| Nitorol® Injection 5 mg | Ampoule | April 30, 1986 |
| Nitorol® Injection 50 mg | Vial | March 15, 1994 |
| Nitorol® Injection 100 mg | Vial | March 15, 1994 |
| Nitorol® Injection Bag 50 mg | Soft bag | February 24, 2005 |
| Nitorol® Injection Bag 100 mg | Soft bag | February 24, 2005 |