- R&D
- Products
- For Print
- May 17, 2023
Eisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito, “Eisai”) today announced the publication of results from a simulation study evaluating the societal value of anti-amyloid-beta (Aβ) protofibril* antibody lecanemab (generic name, U.S. brand name: LEQEMBI™) in individuals living with mild cognitive impairment (MCI) due to Alzheimer’s disease (AD) and mild AD dementia (collectively known as early AD) in the context of the Japanese health care system in the peer-reviewed journal Neurology and Therapy. The paper concluded that lecanemab treatment would improve health and humanistic (quality of life) outcomes and reduce economic burden for individuals with early AD and their caregivers in Japan.
This model-based simulation was conducted using data from the Phase 3 Clarity AD study evaluating the efficacy and safety of lecanemab for early AD with confirmed amyloid pathology by applying an academically validated disease simulation model (AD Archimedes Condition Event simulation: AD ACE model1,2) as well as government statistics, including Japanese epidemiological data and fact-finding surveys on long-term care, and other past research articles to take into account the environment under the Japanese healthcare system from the healthcare payer’s perspective, focusing on direct care costs (including out- and in-patient services, nursing- and home-health- care services, cost of medications, and other intervention costs) and the societal perspective (social costs including informal care costs such as family nursing care in addition to direct care costs). In this paper, the cost reduction effect of lecanemab and the improvement in health outcomes were integrated, incorporating the former cost reduction effect as is, while the latter outcome improvement effect was estimated based on previous studies in the U.S. and the benchmark price estimation process of the U.S. Institute for Clinical and Economic Review (ICER).
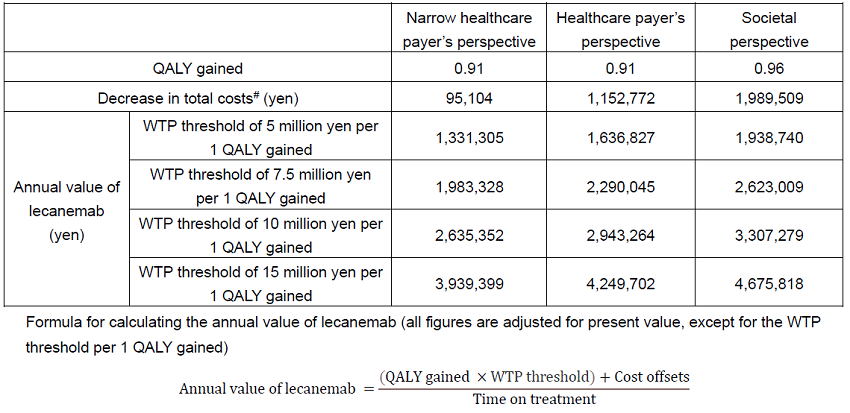
The analysis predicted an increase in Quality-Adjusted Life-Years** (QALYs) by 0.91 from both the narrow healthcare payer’s perspective (considering only medical treatment costs) and the healthcare payer’s perspective (medical treatment and public care costs) (SoC (standard of care***): 6.12, lecanemab+SoC (lecanemab treatment with SoC): 7.03). From the societal perspective, the increase in QALYs was estimated to be 0.96 (SoC: 5.78, lecanemab+SoC: 6.74) compared to SoC. In addition, compared to SoC alone, lecanemab+SoC was predicted to result in a decrease in total costs of 95,104 yen from the narrow healthcare payer’s perspective (SoC: 2,793,491 yen, lecanemab+SoC: 2,698,387: yen), 1,152,772 yen from the healthcare payer’s perspective (SoC: 11,381,044 yen, lecanemab+SoC: 10,228,272 yen), and 1,989,509 yen from the societal perspective (SoC: 24,482,321 yen, lecanemab+SoC 22,492,811 yen). The estimated mean duration of lecanemab treatment was 3.68 years in this simulation.
While taking into account the above factors, the model estimated that the annual value of lecanemab was 1,331,305 yen to 3,939,399 yen from the narrow healthcare payer’s perspective, 1,636,827 yen to 4,249,702 yen from the healthcare payer’s perspective, and 1,938,740 yen to 4,675,818 yen from the societal perspective at the willingness-to-pay (WTP) threshold of 5 million yen to 15 million yen, per 1 QALY gained under the Japanese healthcare system. Regarding the WTP threshold to be paid per 1 QALY gained, an academic article has proposed that 5 times per capita gross domestic product (GDP) is appropriate for severe AD,3 and from an international perspective, it is thought that for common diseases other than AD, the WTP threshold per 1 QALY gained is equivalent to 1-3 times per capita GDP. Considering the above, this paper evaluated that 15 million yen per 1 QALY is appropriate as the WTP threshold for assessing the societal value of lecanemab.
Since AD has a huge impact not only on medical costs but also public care costs as well as the invisible burden including family care, it is important to recognize the societal value, and this paper showed that annual value of lecanemab from the societal perspective can be up to 4,675,818 yen.
Health outcomes (QALYs) and decrease in total costs from lecanemab treatment, and annual value of lecanemab
“Approximately 50% of public long-term care insurance finances in Japan are spent on care costs due to AD (public long-term care insurance finances: 9,626.6 billion yen; public long-term care costs due to AD: 4,783.2 billion yen in 2018). It is understood that AD has a significant impact on not only medical costs but also long-term care insurance finance and the costs due to AD increases significantly with the progression of the pathological stage, and when comparing MCI and severe dementia, medical costs and long-term care costs increase significantly by about 2 times and 10 times, respectively.4 The simulation results show that, in Japan, treatment with lecanemab can have a significant impact on caregivers and society as a whole by improving delaying disease progression”, said Masatomi Akana, Senior Vice President, Chief Government Relations Officer, and Global Value & Access Eisai Co., Ltd., “We believe that this paper will be important information for stakeholders to better understand the potential clinical and socio-economic value of lecanemab in Japan, as well as for discussions on the evaluation of innovation by pharmaceutical companies. We will continue to release data and information transparently and promptly in order to deliver lecanemab to eligible early AD patients.”
“This paper followed the U.S. methodology, and added country-specific conditions to estimate the societal value of the medicine in Japan” said the first author of this paper, Dr. Ataru Igarashi, Associate Professor of Medicine at Yokohama City University and Visiting Associate Professor of Pharmaceutical Policy at the University of Tokyo Graduate School of Pharmaceutical Sciences. “When assessing the value of medicines, not only for dementia but for all diseases, I believe that in addition to efficacy, safety and treatment cost, various other aspects should be taken into account, such as the impact on work and the degree of burden reduction for family members and medical personnel.”
Eisai serves as the lead of lecanemab development and regulatory submissions globally with both companies co-commercializing and co-promoting the product and Eisai having final decision-making authority.
* Protofibrils are large Aβ aggregated soluble species of 75-5000 Kd.5
** The quality-adjusted life year (QALY) is a measure of the value of health outcomes. Since health is a function of length of life (i.e., quantity) and quality of life (QOL), the QALY was developed as an attempt to combine the value of these attributes into a single index number. One QALY equates to one year in perfect health. QOL scores range from 1 (full health) to 0 (dead). For example, if a new treatment and an existing treatment both increase survival years by 3 years, but the new treatment maintains a QOL of 0.7 (QALY=2.1), while the existing treatment has a lower QOL of 0.5 (QALY=1.5), the incremental QALY for the new treatment would be 0.6 (QALY = QOL score x survival years).
*** Standard of Care (SoC) for AD currently consists of lifestyle modifications and pharmacologic treatment of symptoms.
1 Tahami Monfared AA, Tafazzoli A, Ye W, Chavan A, Zhang Q. Long-Term Health Outcomes of Lecanemab in Patients with Early Alzheimer’s Disease Using Simulation Modeling. Neurology and therapy. 2022;11(2):863-80.
2 Tahami Monfared AA, Tafazzoli A, Chavan A, Ye W, Zhang Q. The Potential Economic Value of Lecanemab in Patients with Early Alzheimer’s Disease Using Simulation Modeling. Neurology and Therapy. 2022;11(3):1285-307.
3 Lakdawalla DN, Phelps CE. Health technology assessment with risk aversion in health. J Health Econ. 2020;72:102346. doi: 10.1016/j.jhealeco.2020.102346
4 S. Ikeda, M. Mimura, M. Ikeda, K .Wada-Isoe, M. Azuma, S. Inoue, K. Tomita. Economic Burden of Alzheimer’s Disease Dementia in Japan. Journal of Alzheimer’s Disease 81(2021)309-319
5 Söderberg, L., Johannesson, M., Nygren, P. et al. Lecanemab, Aducanumab, and Gantenerumab — Binding Profiles to Different Forms of Amyloid-Beta Might Explain Efficacy and Side Effects in Clinical Trials for Alzheimer’s Disease. Neurotherapeutics (2022).
Media Inquiries:
Public Relations Department,
Eisai Co., Ltd.
+81-(0)3-3817-5120
Eisai Inc (U.S.)
Libby Holman
201-753-1945
[Notes to editors]
- 1. About Lecanemab
Lecanemab (brand name in the U.S.: LEQEMBI™) is the result of a strategic research alliance between Eisai and BioArctic. Lecanemab is a humanized immunoglobulin gamma 1 (IgG1) monoclonal antibody directed against aggregated soluble (protofibril) and insoluble forms of amyloid-beta (Aβ). In the U.S., LEQEMBI was granted accelerated approval by the U.S. Food and Drug Administration (FDA) on January 6, 2023. LEQEMBI is indicated for the treatment of Alzheimer’s disease (AD) in the U.S. Treatment with LEQEMBI should be initiated in patients with mild cognitive impairment or mild dementia stage of disease, the population in which treatment was initiated in clinical trials. There are no safety or effectiveness data on initiating treatment at earlier or later stages of the disease than were studied. This indication is approved in the U.S. under Accelerated Approval based on reduction in Aβ plaques observed in patients treated with LEQEMBI. Continued approval for this indication may be contingent upon verification of clinical benefit in a confirmatory trial.
Please see full Prescribing Information in the United States.
In the U.S., Eisai submitted a supplemental Biologics License Application (sBLA) to the FDA for approval under the traditional pathway on January 6, 2023. On March 3, 2023, the FDA accepted Eisai’s sBLA based on the Clarity AD clinical data, and the LEQEMBI application has been granted Priority Review, with a Prescription Drug User Fee Act (PDUFA) action date of July 6, 2023. The FDA is planning to hold an Advisory Committee to discuss this application on June 9, 2023. Eisai submitted an application for manufacturing and marketing approval to the Pharmaceuticals and Medical Devices Agency (PMDA) on January 16, 2023, in Japan. The Priority Review was granted by the Ministry of Health, Labour and Welfare (MHLW) on January 26, 2023. Eisai utilized the prior assessment consultation system of PMDA, with the aim of shortening the review period for lecanemab. In Europe, Eisai submitted a marketing authorization application (MAA) to the European Medicines Agency (EMA) on January 9, 2023, and accepted on January 26, 2023. In China, Eisai initiated submission of data for a BLA to the National Medical Products Administration (NMPA) of China in December 2022, and the Priority Review was granted on February 27, 2023. In Canada, Eisai submitted a New Drug Submission (NDS) to Health Canada on March 31, 2023, and was accepted on May 15 of the same year.
Eisai has completed lecanemab subcutaneous bioavailability study, and subcutaneous dosing is currently being evaluated in the Clarity AD (Study 301) OLE.
Since July 2020 the Phase 3 clinical study (AHEAD 3-45) for individuals with preclinical AD, meaning they are clinically normal and have intermediate or elevated levels of amyloid in their brains, is ongoing. AHEAD 3-45 is conducted as a public-private partnership between the Alzheimer’s Clinical Trial Consortium that provides the infrastructure for academic clinical trials in AD and related dementias in the U.S, funded by the National Institute on Aging, part of the National Institutes of Health, Eisai and Biogen.
Since January 2022, the Tau NexGen clinical study for Dominantly Inherited AD (DIAD), that is conducted by Dominantly Inherited Alzheimer Network Trials Unit (DIAN-TU), led by Washington University School of Medicine in St. Louis, is ongoing.
- 2. About the Collaboration between Eisai and Biogen for AD
Eisai and Biogen have been collaborating on the joint development and commercialization of AD treatments since 2014. Eisai serves as the lead of lecanemab development and regulatory submissions globally with both companies co-commercializing and co-promoting the product and Eisai having final decision-making authority.
- 3. About the Collaboration between Eisai and BioArctic for AD
Since 2005, Eisai and BioArctic have had a long-term collaboration regarding the development and commercialization of AD treatments. Eisai obtained the global rights to study, develop, manufacture and market lecanemab for the treatment of AD pursuant to an agreement with BioArctic in December 2007. The development and commercialization agreement on the antibody lecanemab back-up was signed in May 2015.