In drug formulation development, Eisai is committed to maximizing the efficacy of active ingredients and minimizing side effects, while at the same time providing a universal dosage form that is easy to take and use for a wide variety of people, from small children to the elderly. We are constantly reviewing our development methods to deliver valuable formulations to patients as quickly as possible and are also working to provide the highest quality formulations in the shortest period by utilizing digital technologies such as AI and IoT.
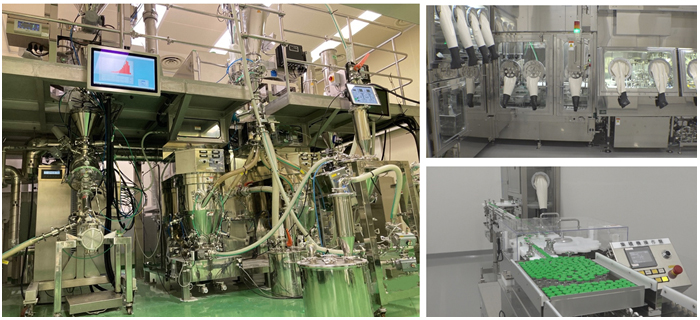
Manufacturing Process DX
The rapid development of high-quality pharmaceutical products (formulations) at low cost is very important in order to deliver medicines to more patients. To provide high quality formulations, we have refined our digital technology to predict and control quality during the manufacturing process. For example, tablets or granules can be irradiated with special light, and the reflected light can be analyzed by a machine learning model to predict the moisture content and concentration of the active ingredient in the tablets or granules.
We have also introduced a continuous manufacturing system, which combines five independent pieces of equipment, to create a highly efficient process that produces granules when raw powder is fed into the equipment, thereby accelerating the development of formulations. In addition, the scale-up experiments normally required for commercial production can be minimized by using a continuous manufacturing system, thus greatly reducing development costs. Tazverik® Tablets (generic name: tazemetostat), which is manufactured at the Kawashima Industrial Park (Gifu, Japan) is the first approved pharmaceutical product in Japan that is manufactured using a continuous manufacturing system.
We have introduced a system that enables us to collect and monitor process data in real time from the above mentioned continuous manufacturing system for the oral formulations, as well as the injectable formulation production line installed at EMITS (Eisai Medicine Innovation Technology Solutions), the newest building at Kawashima Industrial Park. The system is designed to detect and prevent problems in the manufacturing process at an early stage.
Formulation Design DX
The pharmaceutical products (tablets, capsules, granules, etc.) that are prescribed by doctors in everyday life contain several formulation additives in addition to the active pharmaceutical ingredient. Formulation design involves selecting the optimum combination and proportions of active ingredients and formulation additives from an infinite number of combinations. Formulation design is very important because it affects efficacy, safety, and shelf life.
At Eisai, we optimize our formulation design by using digital technology to predict the impact of the formulation on the absorption of the active ingredient in the body. To improve the accuracy of such predictions, we are also investigating the phenomenon of active ingredients dissolving in the body and precipitating back out again after being dissolved, by using special equipment that can mimic the conditions of the human stomach and small intestine. Other digital technology such as the Bayesian optimization algorithm is also being actively used to efficiently find the “best”. Bayesian optimization is a machine learning method of proposing conditions that are likely to yield better results from the data at hand. It supports efficient approaches to reach the best by repeating the cycle of proposal and experimentation. In this way, we aim to deliver medicines to patients as quickly as possible by hybridizing Bayesian optimization without human bias, in addition to the knowledge of experts backed by great experience and history.
