- For Print
- May 28, 2012
Eisai Co., Ltd. (Headquarters: Tokyo, President & CEO: Haruo Naito, “Eisai”) announced today that its U.K subsidiary Eisai Europe Ltd. has received a positive opinion from the European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) for extending the use of once-daily Zonegran® (zonisamide) as monotherapy for the treatment of partial seizures (with or without secondary generalization) in adults with newly diagnosed epilepsy.
Zonegran, originally discovered by Dainippon Sumitomo Pharma Co., Ltd. (formerly Dainippon Pharmaceutical Co., Ltd), was approved in Europe in March 2005 as an adjunctive therapy for the treatment of partial seizures (with or without secondary generalization) in adults with epilepsy, and marketed by Eisai' subsidiaries in Europe.
The CHMP based its decision on clinical data from a double blind, randomized, multicenter study designed to compare once-daily Zonegran with twice-daily controlled release carbamazepine as monotherapy in 583 adults with newly diagnosed partial-onset epilepsy. The study's primary endpoint was the proportion of seizure-free patients at six months. The results of the study showed that Zonegran was effective and well tolerated in newly diagnosed epilepsy patients when used as monotherapy. The statistical comparison between Zonegran and carbamazepine met the criterion of non-inferiority as recommended by treatment guidelines set out by the International League Against Epilepsy (ILAE).
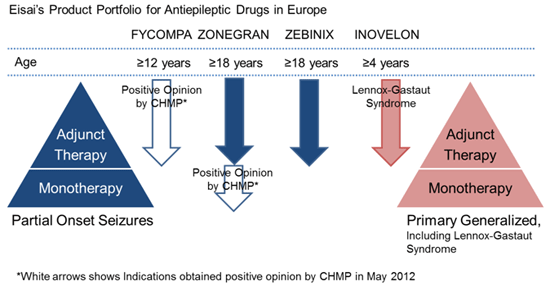
Eisai defines epilepsy as a therapeutic area of focus, in addition to Zonegran, currently marketing Zebinix® (under license from the originator BIAL-Portela & Ca S.A.) as an adjunctive therapy in adults with partial onset seizures, and Inovelon® (under license from the originator Novartis AG) for the adjunctive treatment of seizures associated with Lennox-Gastaut syndrome, a severe form of early childhood-onset epilepsy.
In addition to the CHMP recommendation for the use of Zonegran as a monotherapy, Eisai received a positive CHMP opinion for Fycompa™ (perampanel), AMPA-type glutamate receptor antagonist, as an adjunctive treatment of partial-onset seizures, with or without secondary generalization, in patients with epilepsy aged 12 years and older on May, 2012.
By strengthening its development capabilities and offering multiple treatment options as part of its abundant epilepsy franchise product portfolio, Eisai seeks to make further contributions to address the diversified needs of and increase the benefits provided to epilepsy patients and their families.
[ Please refer to the following note for further information on Eisai's Commitment to Epilepsy ]
Media Inquiries:
Public Relations Department,
Eisai Co., Ltd.
+81-(0)3-3817-5120
< Notes to editors >
1. Eisai's Commitment to Epilepsy
Eisai positions epilepsy as a therapeutic area of focus and currently markets Zonegran® (sodium/calcium channel blocking antiepileptic agent; Europe, the United States, Asia) and Zebinix® (voltage-dependent sodium channel-blocking antiepileptic agent; Europe) as adjunctive therapies in adult patients with partial-onset seizures as well as Inovelon®/BANZEL® (sodium-channel blocking triazole derived antiepileptic agent; Europe, Asia, North America) as an adjunctive therapy for seizures associated with Lennox-Gastaut syndrome, a severe form of childhood-onset epilepsy. Furthermore, Eisai has submitted marketing authorization applications in Europe and the United States seeking approval of its novel AMPA receptor antagonist Fycompa™(perampanel) as a treatment for patients with partial-onset seizures that offers a completely different mechanistic approach to other antiepileptic drugs, and the European application received a positive CHMP opinion in May 2012. By offering multiple treatment options as part of its abundant epilepsy franchise product portfolio, Eisai will make further contributions to address the diversified needs of and increase the benefits provided to epilepsy patients and their families.