- Products
- For Print
- May 22, 2024
Eisai Co., Ltd.
nippon medac Co., Ltd.
Eisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito, “Eisai”) and nippon medac Co., Ltd. (Headquarters: Tokyo, Representative Director and President: Hirohisa Iriyama, “nippon medac”), a subsidiary of medac group (Headquarters: Germany, “medac GmbH”) announced today that they have launched the anti-rheumatic agent “Metoject® Subcutaneous Injection 7.5mg Pen 0.15mL, 10mg Pen 0.20mL, 12.5mg Pen 0.25mL and 15mg Pen 0.30mL” (methotrexate, “MTX”), in Japan. The product received manufacturing and marketing approval in Japan on February 15, 2024, and was published in Japan’s National Health Insurance Drug Price List, today. Based on the license agreement signed by Eisai and medac GmbH in May 2019, nippon medac will hold the marketing authorization of Metoject, while Eisai will be responsible for product distribution of Metoject in Japan.
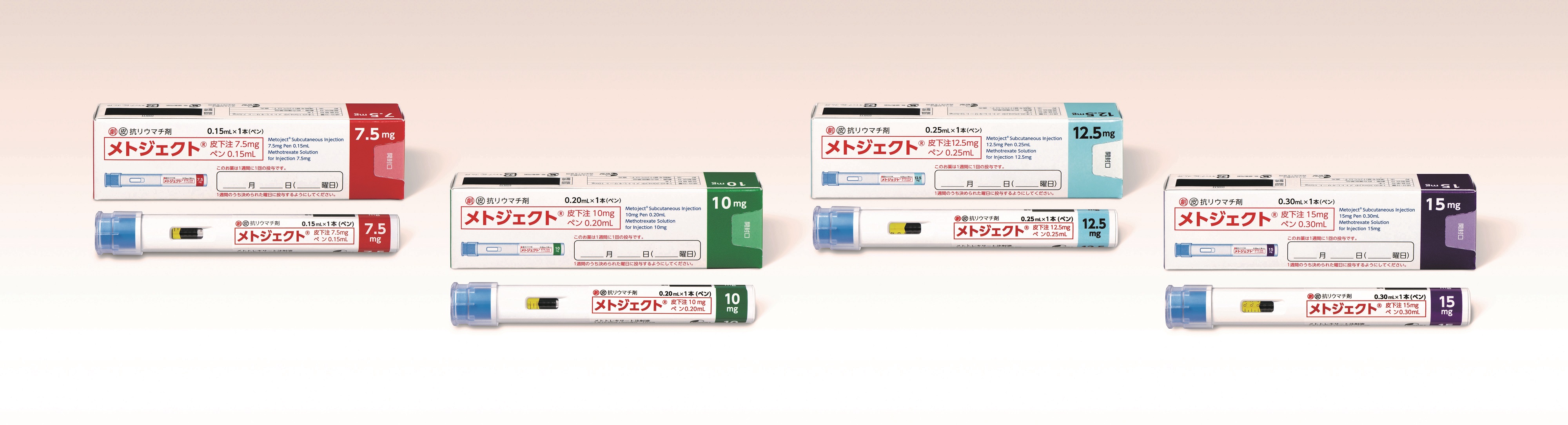
It is estimated that there are approximately 700,000 - 800,000 rheumatoid arthritis patients in Japan,1 and MTX is used as the first-line option for the treatment of rheumatic arthritis. Metoject Subcutaneous Injection Pen will be the first self-administrable MTX subcutaneous injection pen-type autoinjector for rheumatoid arthritis in Japan and was developed to reduce the burden on patients and improve safety during self-injection. The drug can be self-injected in two steps (1- removing the cap, 2- pressing the pen against the skin). The built-in needle cover prevents the needle from being seen before administration and automatically locks after administration to reduce accidental skin puncture risks.
“Eisai has established a solid franchise and has extensive sales experience in the rheumatoid arthritis area in Japan,” said Naoki Sawada, President of Eisai Japan. “By adding Metoject Subcutaneous Injection Pen to Eisai's product lineup, we are making further contributions to address the diversified needs of, and increase the benefits provided to, rheumatoid arthritis patients.”
“Offering Metoject Subcutaneous Injection Pen to rheumatoid arthritis patients as a new treatment option is expected to allow them to practice safe, secure, and easy self-injection,” said Hirohisa Iriyama, Representative Director and President of nippon medac. “nippon medac will contribute to patient-centric rheumatoid arthritis treatment.”
Media Inquiries
-
Eisai Co., Ltd.
Public Relations Department
TEL: +81 (0)3-3817-5120
-
nippon medac Co., Ltd.
Tatsuya Otsuki
TEL: +81-(0)3-6661-6270
[Notes to editors]
- 1. Product Outline in Japan
Product name: Metoject® Subcutaneous Injection 7.5mg pen 0.15mL, 10mg pen 0.20mL, 12.5mg pen 0.25mL and 15mg pen 0.30mL
Generic name: Methotrexate
Indication for use: Rheumatoid arthritis
Dosage and administration: The usual adult dose of methotrexate is 7.5mg injected subcutaneously once a week. The dose may be increased depending on the condition, tolerability, etc. of the patient, but the maximum dosage must not exceed 15 mg.
National Health Insurance (NHI) Drug Price:
Metoject Subcutaneous Injection 7.5mg pen 0.15mL 1,938 JPY per pen
10mg pen 0.20mL 2,310 JPY per pen
12.5mg pen 0.25mL 2,652 JPY per pen
15mg pen 0.30mL 2,972 JPY per pen
Packaging:
Metoject Subcutaneous Injection 7.5mg pen 0.15mL 1 pen
10mg pen 0.20mL 1 pen
12.5mg pen 0.25mL 1 pen
15mg pen 0.30mL 1 pen
- 2. About Metoject Subcutaneous Injection Pen (methotrexate)
Methotrexate (MTX) is positioned as the anchor drug for rheumatoid arthritis treatment.2 For rheumatoid arthritis, it is believed that MTX regulates cell growth by inhibiting folate metabolism in lymphocytes and other cells, and also has an anti-inflammatory mechanism through the promotion of adenosine synthesis in vascular endothelial cells and other cells in synovial membranes. Metoject Subcutaneous Injection Pen will be the first self-administrable MTX subcutaneous injection pen-type autoinjector for rheumatoid arthritis in Japan. It is approved in more than 18 countries in Europe.
- 3. About Eisai
Eisai’s Corporate Concept is “to give first thought to patients and people in the daily living domain, and to increase the benefits that health care provides.” Under this Concept (also known as our human health care (hhc) Concept), we aim to effectively achieve social good in the form of relieving anxiety over health and reducing health disparities. With a global network of R&D facilities, manufacturing sites, and marketing subsidiaries, we strive to create and deliver innovative products to target diseases with high unmet medical needs, with a particular focus in our strategic areas of Neurology and Oncology.
In addition, our continued commitment to the elimination of neglected tropical diseases (NTDs), which is a target (3.3) of the United Nations Sustainable Development Goals (SDGs), is demonstrated by our work on various activities together with global partners.
For more information about Eisai, please visit www.eisai.com (for global headquarters: Eisai Co., Ltd.), and connect with us on X, LinkedIn and Facebook.
- 4. About nippon medac
nippon medac Co., Ltd. was established in April 2016 as the Japanese subsidiary of medac group. We are working to develop new drugs that can expand treatment options for diseases for which therapeutic requirements have not yet been fully met. We will work diligently to deliver quality drugs as quickly as possible to improve the quality of life of patients and their families in areas with high medical needs.
-
1
Report from Study Committee on Rheumatoid Arthritis and Allergy
http://www.mhlw.go.jp/stf/houdou/2r9852000001nfao-att/2r9852000001nfdx.pdf -
2
Japan College of Rheumatology, Clinical practice guideline of methotrexate for patients with rheumatoid arthritis: 2016 updated version
