- For Print
- June 11, 2018
TOKYO, June 11, 2018 – AbbVie GK (Headquarters: Tokyo, President: James Feliciano, “AbbVie”), Eisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito, "Eisai") and Eisai’s subsidiary for gastrointestinal diseases EA Pharma Co., Ltd. (President & CEO: Yuji Matsue; Headquarters: Tokyo, Japan) (hereinafter “EA Pharma”) announced that the autoinjection delivery system for HUMIRA® (generic name: adalimumab [recombinant], “HUMIRA”), HUMIRA® for Subcutaneous Injection 40 mg Pen 0.4 mL and HUMIRA® for Subcutaneous Injection 80 mg Pen 0.8 mL, was listed in the National Health Insurance Drug Price Standard on 30th May. AbbVie and Eisai have launched the products today. HUMIRA is a fully human anti-TNF-α monoclonal antibody formulation.
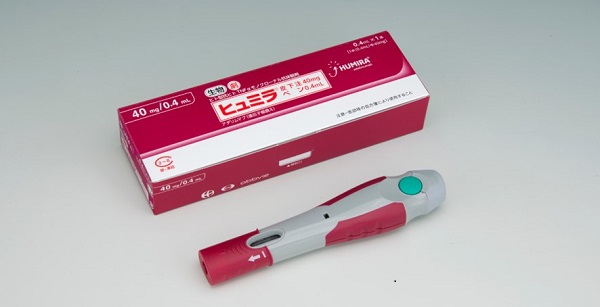
The newly launched pen-type auto-injector devices were developed to simplify the operation and reduce the burden on patients when performing self-injection. The rounded pen-type body of this product is designed to fit in the hands of even patients who have a weak grip and conceal the needle tip when injected. In addition to the lock function and autoinjection delivery system which allows for the full amount drug injection in about 10 seconds, it features injection start and end alert sounds and an inspection window. The pen-type auto-injector devices are filled with the same drug solution as the conventional pre-filled syringe devices*1.
HUMIRA® is the world’s first fully human anti-TNF-α monoclonal antibody and works by neutralizing TNF-α (tumor necrosis factor α), a protein that plays a central role in the inflammatory response of autoimmune diseases such as rheumatoid arthritis. HUMIRA® is already used by one million patients in over one hundred countries.
AbbVie, Eisai and EA Pharma continue to promote and provide information on the proper use of HUMIRA® while making further contributions to improve the quality of life of patients.
-
*1 HUMIRA® for Subcutaneous Injection 40 mg Syringe 0.4 mL and HUMIRA® for Subcutaneous Injection 80 mg Syringe 0.8 mL
About HUMIRA
HUMIRA® is a fully human anti-TNF-α monoclonal antibody formulation. In Japan, it is approved for the indications of “the treatment of rheumatoid arthritis (including inhibition of the progression of structural damage), the treatment of plaque psoriasis, arthritic psoriasis, pustular psoriasis, ankylosing spondylitis, polyarticular juvenile idiopathic arthritis*, intestinal Behçet's disease, and non-infectious uveitis, posterior uveitis or panuveitis, induction and maintenance therapy for moderate to severely active Crohn's disease (limited to patients who have had an inadequate response to conventional therapy), and treatment of moderate to severe ulcerative colitis (limited to patients who have had an inadequate response to conventional therapy ).
*: HUMIRA® for Subcutaneous Injection 80 mg Syringe 0.8 mL and HUMIRA® for Subcutaneous Injection 80 mg Pen 0.8 mL are yet to be approved for this indication.
About AbbVie
AbbVie is a global, research-driven biopharmaceutical company committed to developing innovative advanced therapies for some of the world’s most complex and critical conditions. The company’s mission is to use its expertise, dedicated people and unique approach to innovation to markedly improve treatments across four primary therapeutic areas: immunology, oncology, virology and neuroscience. In more than 75 countries, AbbVie employees are working every day to advance health solutions for people around the world. For more information about AbbVie, please visit us at www.abbvie.com. Follow @abbvie on Twitter, Facebook or LinkedIn.
About Eisai
Eisai Co., Ltd. is a leading global research and development-based pharmaceutical company headquartered in Japan. We define our corporate mission as "giving first thought to patients and their families and to increasing the benefits health care provides," which we call our human health care (hhc) philosophy. With approximately 10,000 employees working across our global network of R&D facilities, manufacturing sites and marketing subsidiaries, we strive to realize our hhc philosophy by delivering innovative products to address unmet medical needs, with a particular focus in our strategic areas of Oncology and Neurology. For further information on Eisai Co., Ltd., please visit www.eisai.com.
About EA Pharma
EA Pharma Co., Ltd., a subsidiary of Eisai Co., Ltd. for gastrointestinal disease area, was established in April 2016 by integration of the gastrointestinal business unit with more than 60 years’ history of the Eisai Group and the gastrointestinal business unit of the Ajinomoto Group having amino acid as its business core. EA Pharma is a gastrointestinal specialty pharma with a full value chain covering R&D, logistics and sales & marketing.
For more information on EA Pharma Co., Ltd., please see www.eapharma.co.jp/en.
Forward-Looking Statements
Some statements in this news release are, or may be considered, forward-looking statements for purposes of the Private Securities Litigation Reform Act of 1995. The words "believe," "expect," "anticipate," "project" and similar expressions, among others, generally identify forward-looking statements. AbbVie cautions that these forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those indicated in the forward-looking statements. Such risks and uncertainties include, but are not limited to, challenges to intellectual property, competition from other products, difficulties inherent in the research and development process, adverse litigation or government action, and changes to laws and regulations applicable to our industry.
Additional information about the economic, competitive, governmental, technological and other factors that may affect AbbVie's operations is set forth in Item 1A, "Risk Factors," of AbbVie's 2016 Annual Report on Form 10-K, which has been filed with the Securities and Exchange Commission. AbbVie undertakes no obligation to release publicly any revisions to forward-looking statements as a result of subsequent events or developments, except as required by law.
###
Media Inquiries
-
AbbVie GK
Public Affairs Division
-
Eisai Co., Ltd.
PR Department
-
EA Pharma Co., Ltd.
Corporate Planning Dept.
