- For Print
- November 29, 2018
EA Pharma Co., Ltd.
Eisai Co., Ltd.
Mochida Pharmaceutical Co., Ltd.
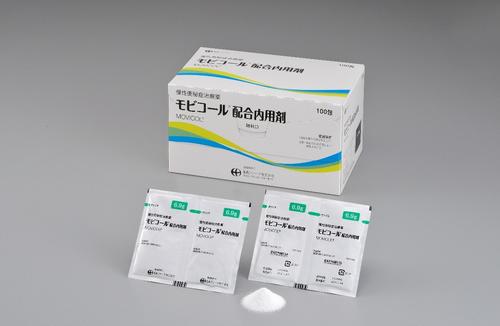
EA Pharma Co., Ltd. (President, Yuji Matsue; Headquarters, Chuo-ku, Tokyo, Japan, “EA Pharma”), EA Pharma’s parent company Eisai Co., Ltd. (CEO, Haruo Naito; Headquarters, Bunkyo-ku, Tokyo, Japan, "Eisai") and Mochida Pharmaceutical Co., Ltd. (President, Naoyuki Mochida; Headquarters, Shinjuku-ku, Tokyo, Japan, “Mochida”) announced today that the constipation treatment “MOVICOL®” (Development code AJG555) was included in Japan’s National Health Insurance drug price list as of November 20, 2018, and EA Pharma and Mochida launched the product onto the Japan market today.
MOVICOL® is the first polyethylene glycol preparation indicated for treatment of chronic constipation1) in Japan, available for adults and children 2 years of age and older. MOVICOL® increases the moisture in the intestinal tract by osmolality of its main ingredient polyethylene glycol (macrogol 4000), which increases fecal moisture, softens feces, increases fecal volume and physiologically activates the peristaltic movement of the colon to promote bowel movement. Furthermore, MOVICOL® is a powdered medicine to be dissolved in water before administration, which enables suitable increase or decrease of the dose to obtain the preferred stool consistency.
Outside Japan, this product has been marketed mainly in Europe by Norgine B.V. (Headquarters, the Netherlands, “Norgine”) under the brand name MOVICOL* and is used by many patients with chronic constipation. In Japan, the Evaluation Committee on Unapproved or Off-Labeled Drugs with High Medical Needs2) of Ministry of Health, Labour and Welfare recognized the “high unmet medical needs” of polyethylene glycol preparations for chronic constipation, and Ajinomoto Pharmaceuticals Co., Ltd. (now EA Pharma) developed this product with Mochida for oral chronic constipation treatment in pediatric and adult patients in Japan under the license granted by Norgine to Ajinomoto Pharmaceuticals Co., Ltd. (now EA Pharma).
In a placebo-controlled double-blind PIII clinical trial in adults conducted in Japan, the MOVICOL® group demonstrated a statistically significant increase in spontaneous bowel movement frequency3) (the primary endpoint) compared to the placebo group. In the subsequent 52-week consecutive administration period, the spontaneous bowel movement frequency was steadily maintained in most cases. In a PIII clinical trial (a baseline-controlled open-label study) in pediatric patients, a statistically significant increase in spontaneous bowel movement frequency (the primary endpoint) was observed compared to baseline. The major adverse drug reactions in these trials were diarrhea and abdominal pain.
EA Pharma and Mochida have commenced distribution of the product under the same brand name in Japan. EA Pharma and Eisai jointly provide information for the proper use of MOVICOL® under a co-promotion agreement.
The prevalence of constipation is high in young women and both elderly men and women, and the symptoms can become severe particularly in pediatric patients. The symptoms of constipation include reduced bowel movement, feeling of incomplete evacuation, and hard stools. When the symptoms become chronic, many patients suffer from a decline of QOL (Quality of Life). With the new treatment MOVICOL® in addition to “GOOFICE® 5 mg Tablet” (bile acid transporter inhibitor for chronic constipation treatment) launched in Japan in April 2018, EA Pharma, Eisai and Mochida are striving to increase treatment options for patients with chronic constipation and diverse disease histories as they seek to further contribute to addressing the needs of, and increasing the benefits provided to, patients, their families and healthcare providers.
-
1)
Excluding structural disease-induced constipation
-
2)
The Evaluation Committee on Unapproved or Off-Labeled Drugs with High Medical Needs
This committee was organized in the Ministry of Health, Labour and Welfare to evaluate the medical needs of the drugs and indications that are not approved in Japan (“unapproved or off-labeled drugs”) and the appropriateness of filing the public knowledge-based application and requirement of additional studies for filing the marketing and manufacturing application to promote development of unapproved or off-labeled drugs by pharmaceutical companies. -
3)
Defecation without use of laxative, enema or manual disimpaction
End
Media Inquiries
-
EA Pharma Co., Ltd.
Corporate Planning Dept.
TEL: +81(0)3-6280-9802 -
Eisai Co., Ltd.
PR Dept.
TEL: +81(0)3-3817-5120 -
Mochida Pharmaceutical Co., Ltd.
Public Relations
TEL: +81(0)3-3225-6303
More Information
1. MOVICOL® Product Outline
*You can scroll to the left or right here
| Brand name | MOVICOL® |
| Ingredients |
macrogol 4000, sodium chloride, sodium bicarbonate, potassium chloride |
| Formulation, contents |
Powdered preparation for oral fluid
|
| Indication | Chronic constipation (excluding structural disease-induced constipation) |
| Dosage and administration |
Dissolve this powdered medicine in water and administer orally. For children aged between 2 and 6 years, normally 1 sachet should be administered orally at once per day for the initial dose. Then, the dose can be increased or decreased depending on symptoms, administered orally 1-3 times per day. The maximum daily dose is 4 sachets (up to 2 sachets at once). An interval of at least 2 days is needed between every dose escalation, and the dose should not be increased in excess of 1 sachet per day. For children aged between 7 and 11 years, normally 2 sachets should be administered orally at once per day for the initial dose. Then, the dose can be increased or decreased depending on symptoms, administered orally 1-3 times per day. The maximum daily dose is 4 sachets (up to 2 sachets at once). An interval of at least 2 days is needed between every dose escalation, and the dose should not be increased in excess of 1 sachet per day.
|
| Packaging | 100 sachets |
| National Health Insurance Drug (NHI) Price | MOVICOL® 6.8523g 1 sachet \83.90 |
| Date of manufacture and marketing approval | September 21, 2018 |
| Date of inclusion in the NHI drug price list | November 20, 2018 |
| Launch | November 29, 2018 |
| Manufacturer and distributor | EA Pharma Co., Ltd. |
| Promotion alliance with EA Pharma Co., Ltd. | Eisai Co., Ltd. |
| Distributor | Mochida Pharmaceutical Co., Ltd. |
2. About “GOOFICE® 5 mg Tablet”
“GOOFICE® 5 mg Tablet”, which EA Pharma in-licensed from Albireo AB (Headquarters, Sweden), is an orally available chronic constipation** treatment having a novel mechanism of action. “GOOFICE® 5 mg Tablet” inhibits the bile acid transporter that regulates reabsorption of bile acids thereby increasing the flow of bile acids to the colon, which is expected to enhance colonic motility. EA Pharma and Mochida have a joint development and marketing agreement for “GOOFICE® 5 mg Tablet”, and started distribution of “GOOFICE® 5 mg Tablet” respectively under the same brand name on April 19, 2018 in Japan. EA Pharma also has a co-promotion agreement with Eisai. EA Pharma and Eisai jointly provide information for the proper use of “GOOFICE® 5 mg Tablet”.
**Excluding structural disease-induced constipation
3. About EA Pharma Co., Ltd.
EA Pharma Co., Ltd., a subsidiary of Eisai Co., Ltd. for gastrointestinal disease area, was established in April 2016 by integration of the gastrointestinal business unit with more than 60 year’s history of the Eisai Group and the gastrointestinal business unit of the Ajinomoto Group having amino acid as its business core. EA Pharma is a gastrointestinal specialty pharma with a full value chain covering R&D, logistics and sales & marketing.
For more information on EA Pharma Co., Ltd., please see http://www.eapharma.co.jp/en/
4. About Eisai Co., Ltd.
Eisai Co., Ltd. is a leading global research and development-based pharmaceutical company headquartered in Japan. We define our corporate mission as "giving first thought to patients and their families and to increasing the benefits health care provides," which we call our human health care (hhc) philosophy. With approximately 10,000 employees working across our global network of R&D facilities, manufacturing sites and marketing subsidiaries, we strive to realize our hhc philosophy by delivering innovative products to address unmet medical needs, with a particular focus in our strategic areas of Oncology and Neurology.
For more information on Eisai Co., Ltd., please see https://www.eisai.com/
5. About Mochida Pharmaceutical Co., Ltd.
Mochida Pharmaceutical Co., Ltd. has been committed to research and development of innovative pharmaceutical products since its establishment thereby providing distinctive medicines to the medical field. Currently, the core pharmaceutical business focuses resources on the targeted areas of cardiovascular medicine, obstetrics and gynecology, dermatology, psychiatry and gastroenterology, while also providing medicine for intractable disease as well as generics including biosimilars, to meet medical needs.
For more information on Mochida Pharmaceutical Co., Ltd., please see http://www.mochida.co.jp/english/
*“MOVICOL” is a registered trademark of the Norgine group.
(In this document, MOVICOL means the product marketed in non-Japan countries.)