




Dr. Hachiro Sugimoto
Born in 1942. Joined Eisai in 1961. Leader of E2020 (Aricept) project team at Brain and Nerve Unit Chemistry Group, Tsukuba Research Laboratories (TRL) from 1983. Aricept creators representative. Previous positions in Eisai include Director of Discovery Research Laboratory I. Professor at Graduate School of Pharmaceutical Sciences, Kyoto University in 2003. Currently conducting further research on Alzheimer's disease as Chair Professor of Center for Neurologic Disease, Graduate School of Brain Science, Doshisha University. Current chairman of venture business PharmaEight Co., Ltd.
Dr. Sugimoto : My mother developed dementia in her later years. Whenever I went to visit her, she'd ask me, “Young man, who are you?” It was a shock to me that my mother could no longer recognize her own son. I would say to her, “Mom, it's me, your son Hachiro,” and she'd reply, “Oh, you don't say? My son's name is Hachiro. You both have the same name.” It was a very sad time for me and I found it difficult to accept the reality of the situation. With these memories of my mother playing in my mind, I felt inspired that it was my mission as a scientist to generate an effective new drug for Alzheimer's, despite the illness' notoriety for being one of the most difficult-to-treat diseases.
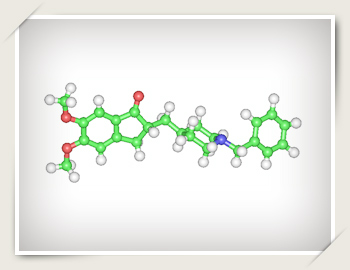
Structural formula of donepezil

Dr. Sugimoto : My mother eventually passed away from dementia-related causes and that had a huge personal impact on me. Being a scientist who works in a pharmaceutical company by profession, I knew then that I wanted to create a drug for treating Alzheimer's disease.
Research for Aricept first began in 1983. At the time, there was a hypothesis that suggested that neurotransmitters in the brain called acetylcholines were closely linked to abnormal decreases in memory function in patients with Alzheimer's disease. It was stubborn perseverance on our part in regard to the acetylcholine deficiency hypothesis that ultimately enabled us to bring the project to fruition.
Dr. Sugimoto : During the time when I was the leader of the E2020 (Aricept) project team, the acetylcholine deficiency hypothesis had already gained a certain level of recognition, but there were also opposing views that proposed that the hypothesis could turn out to be a failure.
The discovery of our seed compound was by mere coincidence. We came across the compound during random screening when searching for compounds that possessed inhibitory activity for acetylcholinesterase, which is an enzyme that breaks down acetylcholine. The compound had previously been synthesized with a different purpose in mind but, surprisingly, it ended up leading the way to the creation of Aricept.
Next, we further developed our seed compound through chemical synthesis and in 1985 generated a new lead compound with still-higher efficacy. Unfortunately, the compound was later found to have problems related to its pharmacokinetic profile such as low bioavailability, but after further attempts to overcome this issue, we were able to successfully create E2020 (Aricept) with a promising enough profile for the compound as a drug candidate. In March 1987, Eisai formally decided that the project would take the next step in its development.

Eisai's Tsukuba Research Laboratories, where Aricept was discovered

Dr. Sugimoto : Back then, the Tsukuba Research Laboratories were organized by disease field, with six laboratories in total. This helped to encourage a healthy sense of competition among the laboratories.
As for us, we worked at the Brain and Nerve Unit in Laboratory II, which consisted of the Chemistry Group and Biology Group. The two groups would sometimes get into heated discussions, almost arguments. This was because the Chemistry Group would synthesize as many compounds as possible and submit them to the Biology Group for evaluation—with the Biology Group's resources being limited, members from both teams could feel frustrated with the process at times.
That said, the current Eisai president & CEO, Haruo Naito, who was a senior director of the Tsukuba Research Laboratories then, would stop by every evening to offer us words of encouragement. The result was often that our morale would soon pick up again—we all wanted the project to succeed—and so we'd end up working until late at night.
Dr. Sugimoto : When we're seeking to create new drugs, it's more often than not that an individual's “serendipity” is what initially gets the ball rolling.
E2020 was no exception—at the time, our chemistry team was facing a situation in which they were saturated with “SAR,” or “structure–activity relationship” information, through the research activities they had engaged in so far. One bright idea, however, was had by one of our newcomers at the time, who had only joined the company less than a year earlier. It was the “serendipity” of this young researcher that enabled him to successfully synthesize the final compound, E2020.
Of course, the credit doesn't just go to one individual. There is no mistake that it was each researcher on the project performing their respective role that enabled us to achieve results. It goes without saying that the ability of each individual is also crucial, but teamwork is no less vital. To create innovative new drugs, I believe that it's not only the talents of individual researchers but how well they complement each other as a whole that leads to greater success.

E2020 project members

Dr. Sugimoto speaking at an Aricept launch event in February 1997 in Atlanta, the United States. He was welcomed by the audience with a standing ovation.

Dr. Sugimoto : Looking back on other Eisai products that have gone on to become blockbuster drugs, such as Juvela Nicotinate, Neuquinon and Methycobal, you could say that expectations during the R&D phase for those drugs were actually on the chilly side. In any drug development, you see, it's quite difficult for things to simply sail smoothly from day one.
With new drug development, you'll often have one person who has stuck to a particular idea very passionately—even obsessively. I think that one key success factor is that that person is provided support both explicitly and implicitly from top management and all teams involved in the project. The same applied to E2020—top management was there to keep an optimistic eye over things to balance out the more reserved expectations from others on the whole.
Dr. Sugimoto : Aricept is a gold-standard drug as an acetylcholinesterase inhibitor and possesses an established safety profile, so I would love to see Aricept out there benefitting as many patients as possible worldwide in order to maximize its full potential.
I am proud of Eisai's achievements in being able to contribute to the quality of life of patients and their families around the world as a pioneer in the treatment of Alzheimer's disease. I hope that Eisai will continue to develop drugs beyond Aricept to treat the underlying cause of the disease as well to follow through on the responsibilities that come with the hard-earned reputation that it has built in the industry.