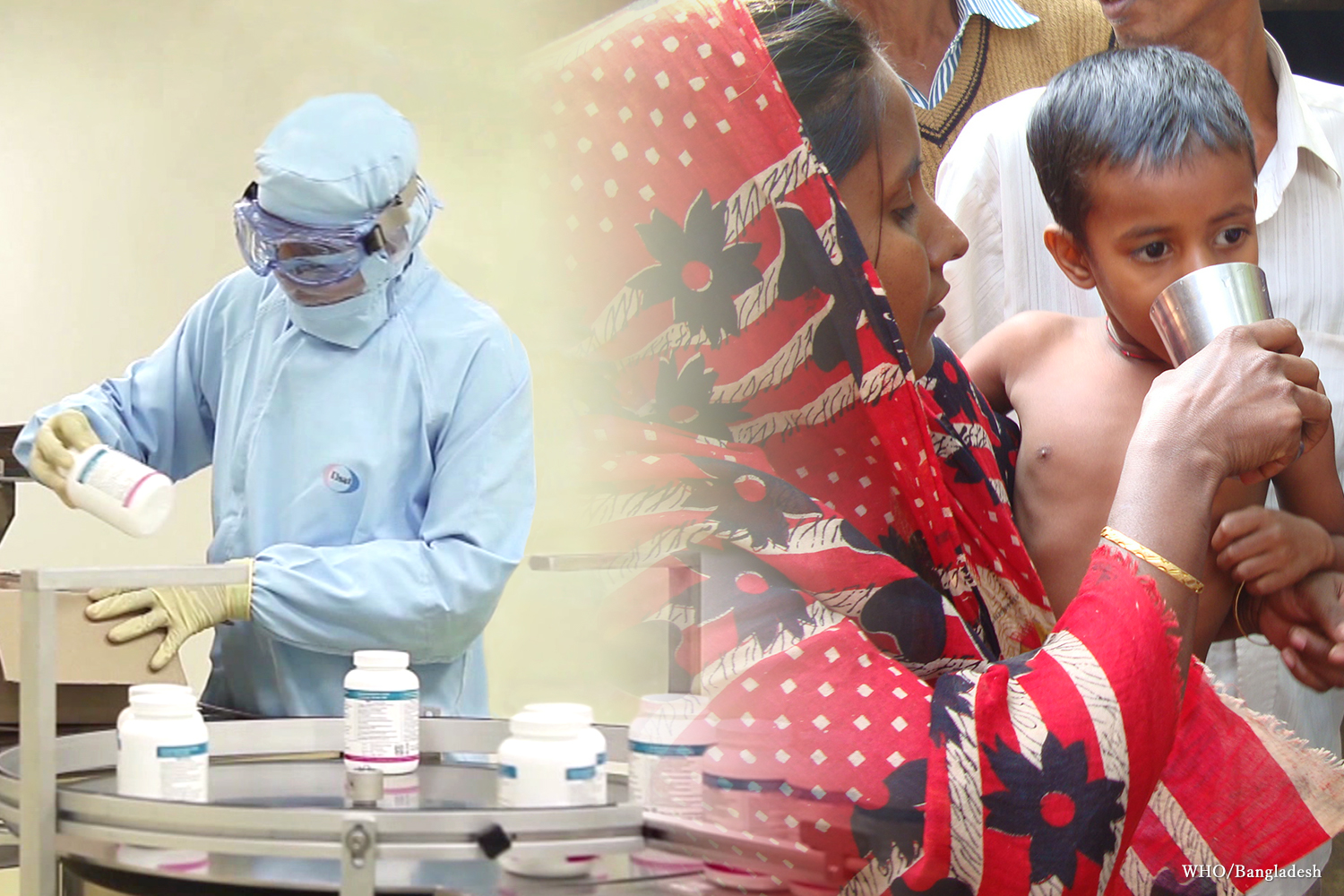
Our Concept for Access to Medicines
There are 2 billion people in the world who lack access to essential health care and medicines. Along with governments, international organizations such as the World Health Organization (WHO), nonprofit organizations (NPOs), non-government organizations (NGOs), and other private sector companies, Eisai is determined to play a central role in improving access to medicines worldwide through public-private partnerships.