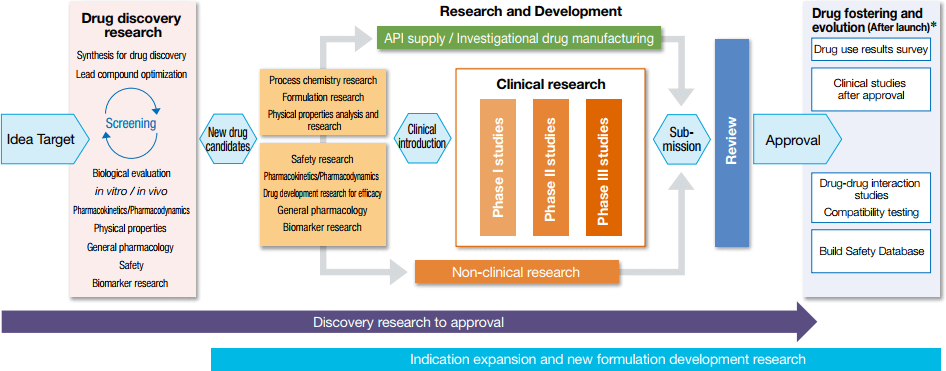
As the name literally suggests, drug creation research is research into producing new drugs, which can be broken down into three stages: drug discovery research, drug development research and clinical research. During drug discovery research, researchers employ state-of the-art technology to screen for and identify highly effective compounds and also conduct basic research using external resources. The identified drug candidates then proceed to the drug development stage, where researchers lay the groundwork for filing and approval around the world by evaluating the compounds' physicochemical and biological properties, assuring their quality and safety, and performing process chemistry research into methods of large-scale synthesis and manufacturing. Drug candidates that clear development research are then elevated to the clinical research stage. After three phases of clinical trials (Phase I, Phase II, and Phase III), drugs candidates that are approved by regulatory authorities can be launched on the market.
- *Post-launch research is carried out to pursue more appropriate drug usage, various improvements and new drug development.


