- For Print
- July 27, 2012
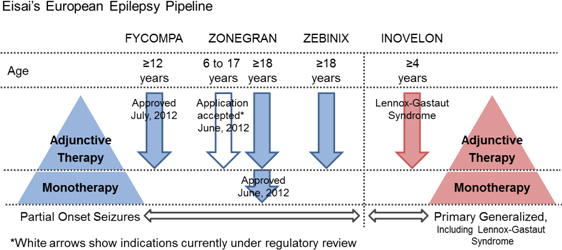
Eisai Co., Ltd. (Headquarters: Tokyo, President & CEO: Haruo Naito, “Eisai”) announced today its U.K. subsidiary Eisai Europe Limited has received approval from the European Commission (EC) to market the AMPA receptor antagonist Fycompa® (perampanel) as an adjunctive treatment of partial-onset seizures, with or without secondarily generalized seizures, in people with epilepsy aged 12 years and older.
Discovered and developed by Eisai, Fycompa is a highly selective, non-competitive AMPA-type glutamate receptor antagonist. Epileptic seizures are primarily mediated by the neurotransmitter glutamate. As an AMPA receptor antagonist, Fycompa reduces neuronal hyperexcitation associated with seizures by targeting glutamate activity at post-synaptic AMPA receptors. This mechanism of action, which is different to that of currently marketed antiepileptic drugs (AEDs), means that Fycompa is the first approved AED in this new class of treatment. Approval by the EC for use in both adults and adolescents 12 years and older, Fycompa delivers the benefit of once-daily oral dosing, and thereby it may help reduce the potential pill-burden a patient with epilepsy may experience as well as improve patient drug compliance. Eisai plans to launch Fycompa successively across the European Union(EU) so as to bring this new treatment to patients as early as possible.
The EC based its approval decision on clinical data from three pivotal Phase III, global, randomized, double-blind, placebo-controlled, dose-escalation studies which examined 1,480 people with partial epilepsy. Each of the studies showed consistent results in the efficacy and tolerability of perampanel as an adjunctive therapy in people with partial-onset seizures (with or without secondary generalization). The most commonly reported adverse events were dizziness, headache, somnolence, irritability, fatigue, falls, and ataxia.
There are an estimated six million people living with epilepsy in Europe, and more than 50 million people worldwide. Eisai defines epilepsy as a therapeutic area of focus, with its currently marketed European epilepsy portfolio comprising Zonegran® (under license from the originator Dainippon Sumitomo Pharma Co., Ltd.) and Zebinix® (under license from the originator BIAL-Portela & Ca S.A.) as adjunctive therapies in adult epilepsy patients with partial-onset seizures, and Inovelon® (under license from the originator Novartis AG) for the adjunctive treatment of seizures associated with Lennox-Gastaut syndrome, a severe form of early childhood-onset epilepsy. By enhancing its epilepsy drug development capabilities and providing multiple treatment options as part of an abundant epilepsy product portfolio, Eisai seeks to make further contributions to address the diversified needs of, and increase the benefits provided to, epilepsy patients and their families.
[ Please refer to the following notes for further information on epilepsy, Fycompa (perampanel), perampanel Phase III studies, and Eisai's Commitment to Epilepsy ]
Media Inquiries:
Public Relations Department,
Eisai Co., Ltd.
+81-(0)3-3817-5120
< Notes to editors >
1. About Epilepsy
Epilepsy is a medical condition that produces seizures affecting a variety of mental and physical functions. A patient is considered to have epilepsy after two or more unprovoked seizures. A seizure occurs when a brief, strong surge of electrical activity affects part or all of the brain. An individual can have various symptoms, from convulsions and loss of consciousness, to some that are not always recognized as seizures, such as blank staring, lip smacking, or jerking movements of arms and legs.
Epilepsy can develop at any age and 0.5% to 2% of people will develop epilepsy during their lifetime. Epilepsy reportedly affects nearly 1 million people in Japan, 2.4 million people in Europe (G5), 3 million people in the United States, and more than 50 million people worldwide. Epilepsy constitutes an area in which there are significant unmet medical needs, with up to a third of people with partial epilepsy in Europe not achieving seizure freedom despite therapy with anti-epileptic drugs.
2. About AMPA Receptor Antagonist Fycompa® (perampanel)
Fycompa® (perampanel), a novel chemical entity discovered and in development by Eisai, is a highly selective, non-competitive AMPA-type glutamate receptor antagonist. Perampanel is the first anti-epileptic treatment to reduce neuronal hyperexcitation associated with seizures by targeting glutamate activity at post-synaptic AMPA receptors. Perampanel has demonstrated broad-spectrum anti-seizure effects in Phase II and III studies. The agent is approved in Europe for the treatment (once-daily oral dose) of partial-onset seizures, with a New Drug Application (NDA) currently under review in the United States. Perampanel is also being evaluated in a Phase III study in Japan. Furthermore, Eisai is conducting a global Phase III study for generalized epilepsy and plans to conduct further studies to evaluate the agent as a monotherapy in the treatment of partial-onset seizures, Lennox-Gastaut syndrome and other forms of epilepsy as it seeks to expand the range of indications for which the drug is approved.
3. About Perampanel Phase III studies
The clinical development plan for perampanel consisted of three global Phase III studies (Studies 306, 305 and 304) in which a total of 1,480 epilepsy patients aged 12 years and older participated. The key goal of Study 306 was to identify the minimal effective dose and included four treatment arms (placebo, 2mg, 4mg, and 8mg). Studies 304 and 305 included three arms (placebo, 8mg, and 12mg) and were to evaluate a more extended dose range.
The studies were similar in design: global, randomized, double-blind, placebo-controlled, dose-escalation, parallel-group studies. The primary and secondary endpoints were the same in all the studies: percentage change in seizure frequency, 50% responder rate, percentage reduction of complex partial plus secondarily generalized seizures, and evaluation for dose response. The primary endpoint for the EMA is 50% responder rate and the FDA is median percent change in seizure frequency. Specifically the results showed:
Study 306
- The 50% responder rates compared to placebo for the ITT (intention-to-treat) population were: 20.6% (p=0.4863), 28.5% (p=0.0132), and 34.9% (p=0.0003) in the 2, 4, and 8 mg perampanel/day groups, respectively, versus 17.9% with placebo.
- The median percent change in seizure frequency for the ITT population shown: 2 mg = -13.6% (p=0.4197), 4 mg = -23.3% (p=0.0026), 8 mg = -30.8% (p<0.0001) and placebo = -10.7%
- The most frequent treatment-emergent adverse events were dizziness, headache and somnolence.
Study 305
- The 50% responder rates compared to placebo for the ITT (intention-to-treat) population were:, 33.3% (p=0.0018), and 33.9% (p=0.0006) in the 8 and 12 mg perampanel/day groups, respectively, versus 14.7% with placebo.
- The median percent change in seizure frequency for the ITT population shown: 8mg = -30.5% (p=0.0008), 12mg = -17.6% (p=0.0105) and placebo = -9.7%
- The most reported adverse events were dizziness, fatigue, headache and somnolence
Study 304
- The 50% responder rates compared to placebo for the ITT (intention-to-treat) population were: 37.6% (p=0.0760), and 36.1% (p=0.0914) in the 8 and 12 mg perampanel/day groups, respectively, versus 26.4% with placebo.
- The median percent change in seizure frequency for the ITT population shown: 8mg = -26.3% (p=0.0261), 12mg = -34.5% (p=0.0158) and placebo = -21.0%
- The most common side effects were dizziness, somnolence, irritability, headache, falls, and ataxia.
4. Eisai's Commitment to Epilepsy
Eisai defines epilepsy as a therapeutic area of focus, not only developing the AMPA receptor antagonist perampanel globally, but currently marketing Zonegran® (under license from the originator Dainippon Sumitomo Pharma Co., Ltd.; sodium/calcium channel blocking antiepileptic agent; marketed in Europe, the United States and Asia) and Zebinix® (under license from the originator BIAL-Portela & Ca S.A.; voltage-dependent sodium channel-blocking antiepileptic agent; marketed in Europe) as adjunctive therapies in adults with partial-onset seizures, and Inovelon®/BANZEL® (under license from the originator Novartis AG; sodium channel-blocking novel triazole derived antiepileptic agent; marketed in Europe, Asia (Inovelon®), and the North America (BANZEL®)) for the adjunctive treatment of seizures associated with Lennox-Gastaut syndrome, a severe form of early childhood-onset epilepsy.