- For Print
- March 9, 2011
Eisai Co., Ltd.
Sanko Junyaku Co., Ltd.
Eisai Co., Ltd. (Headquarters: Tokyo, President & CEO: Haruo Naito, “Eisai”) and its diagnostics subsidiary Sanko Junyaku Co., Ltd. (Headquarters: Tokyo, President & CEO: Keisuke Watanabe, “Sanko Junyaku”) announced today that the two companies will launch the Cobas h 232 Series (“the Series”), a medical device series manufactured and marketed by Roche Diagnostics K.K. (Headquarters: Tokyo, President & CEO: Ayumu Ogawa, “Roche”), on April 5. Eisai will serve as co-promotion partner in the marketing of the Series.
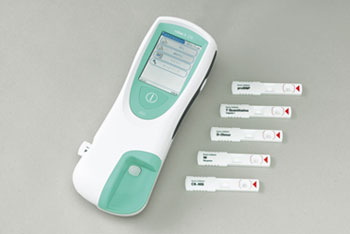
Marketed by Roche since July 2007, the Series is a Point-of-Care Testing (POCT) system that enables rapid determination of five kinds of cardiac blood markers associated with heart failure, acute myocardial infarction, pulmonary thromboembolism and other cardiovascular conditions. Sanko Junyaku will begin marketing the Series based on a licensing agreement it concluded with Roche on March 1, 2011.
Cardiovascular emergency patients usually undergo a range of screening tests such as electrocardiogram (ECG) testing, diagnostic imaging and blood tests. The use of the Series to conduct blood tests aids in the early diagnosis of cardiovascular emergencies and allows physician’s to assess a patient's condition. The main unit is portable and compact which allows measurements to be taken in a variety of settings such as the physician's office, hospital outpatient clinics or hospital wards as well as in emergency situations.
With a view to further fulfilling its corporate mission of human health care (hhc), the Eisai Group established a new Eisai Japan organizational structure in June 2010 to formulate and implement comprehensive strategies across its four Japan business segments comprising prescription pharmaceuticals, consumer healthcare products, diagnostics, and generics. Furthermore, Eisai currently markets a number of cardiovascular products such as the thrombolytic agent Cleactor®, a treatment for acute myocardial infarction and pulmonary thromboembolism, Coretec®, a treatment for acute heart failure, and the Nitorol® Series, for treatment of acute heart failure and ischemic heart disease. By providing a comprehensive range of information and products related to all aspects of cardiovascular care, from screening through treatment, Eisai and Sanko Junyaku seek to make further contributions to increasing the benefits provided to patients.
*Sanko Junyaku Co., Ltd. will change its corporate name to Eidia Co., Ltd., effective April 1.
[ Please refer to the following notes for a product outline, product features, and product image ]
Media Inquiries:
-
Eisai Co., Ltd.
Public Relations Department,
-
Sanko Junyaku Co., Ltd.
Public Relations Section,
General Affairs Department,
< Notes to editors >
 Product Outline (Main System Components)
Product Outline (Main System Components)
1. Medical Device (General Medical Device;Specially Controlled Medical Device)
| Product Name | Cobas h 232 Meter | Cobas h 232 Meter Scanner-Version (with integrated barcode scanner) |
|---|---|---|
| Dimensions | 275 x 102 x 55 (mm) | |
| Weight | approx. 650g (incl. POCT handheld battery pack) | |
| Measurement Time | 8-12 minutes (depending on test parameters) | |
| Sample Material | Heparinized venous whole blood | |
| Sample Size | 150µL whole blood | |
| Memory | 500 test results (with date, time and comments) | |
| Suggested Retail Price | 500,000 yen | 600,000 yen |
| Manufactured and Marketed By: Roche Diagnostics K.K. Distributed By: Eidia Co., Ltd. (Currently Sanko Junyaku Co., Ltd.) Co-promoted By: Eisai Co., Ltd. |
||
|
CARDIAC Syringe | 1,300 yen |
|---|---|---|
| POCT Handheld Battery Pack | 24,000 yen | |
| POCT Handheld Base Unit | 80,000 yen | |
| CARDIAC Reader IQC (Used to control performance of the meter's optical system) | 3,700 yen |
2. Reagents (In-vitro Diagnostics)
| Product Name | Related Condition | Tests Per Package |
Suggested Price (Tax Exclusive) |
|---|---|---|---|
| CARDIAC Troponin T | Myocardial Damage | 10 Tests | 18,000 yen |
| CARDIAC D-Dimer | Pulmonary Thromboembolism |
10 Tests | 18,000 yen |
| CARDIAC NT-proBNP | Heart Failure | 10 Tests | 22,000 yen |
| CARDIAC CK-MB | Myocardial Infarction | 10 Tests | 15,000 yen |
| CARDIAC Myoglobin | Myocardial Infarction | 20 Tests | 30,000 yen |
|
Manufactured and Marketed By: Roche Diagnostics K.K. Distributed By: Eidia Co., Ltd. (Currently Sanko Junyaku Co., Ltd.) Co-promoted By: Eisai Co., Ltd. |
|||
- *Sold Separately: Control sets for use with each of the respective CARDIAC reagents (5 types)
(Reference)Test Fees:
| Product Name | Test Fee | Test Determination Fees |
|---|---|---|
| CARDIAC Troponin T | 130 Points | 144 Points (Biochemical Test Determination Fee (I)) |
| CARDIAC D-Dimer | 150 Points | 125 Points (Blood Test Determination Fee) |
| CARDIAC NT-proBNP | 140 Points | 144 Points (Biochemical Test Determination Fee (II)) |
| CARDIAC MyoglobinP | 150 Points | 144 Points (Biochemical Test Determination Fee (I)) |
| CARDIAC CK-MB | 90 Points | 144 Points (Biochemical Test Determination Fee (I)) |
 Product Features
Product Features
- Measures N-terminal pro B-type natriuretic peptide (NT-proBNP), Troponin-T, D-Dimer and other cardiac blood markers in only 8 to 12 minutes
- Measurement is possible with 150µL of heparinized venous whole blood
- Its compact size and use of handheld battery pack (sold separately) make it easily portable
- Employs use of a touch-screen and is conveniently designed to be operated by following on-screen instructions
- Stores results from a maximum of 500 tests
- Lists all test results of individual patients
 Glossary of Terms
Glossary of Terms
1. POCT (Point-of Care Testing)
Point-of Care Testing is defined as medical testing that is able to be performed rapidly and easily anywhere including at a patient’s bedside, at the physician’s office, or in emergency situations. This kind of testing allows for on-the-spot determination of results.
2. Troponin T
Troponin T is one of a number of proteins that make the heart and other muscles. Elevated concentrations of Troponin T tend to be present following damage to the myocardium. Troponin T is useful in the diagnosis of myocardial infarction and is a marker that has a high specificity for myocardial damage.
3. D-Dimer
D-Dimer is a substance that is produced during the breakdown of blood clots. Whereas elevated concentrations of D-Dimer tend to be present following the formation of a blood clot somewhere in the body, normal levels of D-Dimer are an indication that blood clot formation may be ruled out. D-Dimer is also reported to be useful in exclusion diagnosis as clinical symptoms in patients with pulmonary thromboembolisms are the same as those that appear in patients with cardiovascular diseases. It is also known as D-DDimer.
4. NT-proBNP (N-Terminal pro B-type Natriuretic Peptide)
NT-proBNP is a substance that is produced by myocardial cells and released into the blood in response to excessive strain on the heart muscle. This marker is used to rule out heart failure and in the assessment of a patient’s condition.
5. Myoglobin
Myoglobin is a protein found in the heart and other muscles. Elevated concentrations of myoglobin tend to be present when damage is sustained by the heart muscle. Myoglobin is useful in the early diagnosis of myocardial infraction.
6. CK-MB (MB Creatine Kinase Isoenzyme)
CK-MB is an enzyme found in large quantities in the heart muscle. Elevated CK-MB levels tend to be present when damage is sustained by the heart muscle. CK-MB is useful in the diagnosis of myocardial infarction and assessment of a patient’s condition.
 Product Photograph
Product Photograph