- For Print
- September 2, 2010
Eisai Co., Ltd. (Headquarters: Tokyo, Japan, President & CEO: Haruo Naito, “Eisai”) announced today the start of the first patient enrolled clinical study with BAN2401, a novel monoclonal antibody that is being developed as a potential next-generation therapeutic treatment for Alzheimer's disease. BAN2401 is the first monoclonal antibody to selectively bind, neutralize and eliminate soluble protofibrils, the toxic amyloid-β
aggregates thought to cause Alzheimer's disease. With this new treatment approach, it is feasible to expect that BAN2401 may have the potential to halt progression of the disease.
Eisai obtained the global rights to study, develop, manufacture and market BAN2401 for the treatment of Alzheimer's disease through an agreement it concluded with BioArctic Neuroscience AB (Headquarters: Stockholm, Sweden, CEO: Dr. Pär Gellerfors, “BioArctic Neuroscience”) in December 2007.
The study (Phase I) will primarily evaluate BAN2401 to determine safe dose range and its effects on Alzheimer biomarkers in more than 80 Alzheimer's patients with mild to moderate forms of the disease.
The role of monoclonal antibody therapies in treating medical conditions has expanded tremendously since the first such therapy was developed in the 1970s. In particular, their use in treating neurological conditions has increased in the past few years. The commencement of the first BAN2401 clinical study marks a huge step forward in the creation of a new drug therapy that uses monoclonal antibodies as an approach to treating Alzheimer's disease. If clinical development progresses smoothly, Eisai intends to submit regulatory applications for approval of BAN2401 in 2015 or 2016.
Since pioneering the establishment of a therapeutic treatment for Alzheimer's disease with the launch of Aricept® (generic name: donepezil hydrochloride) 5 mg and 10 mg tablets, Eisai has gone on to provide a more beneficial treatment option with the development of the higher-dose Aricept® 23 mg tablet. By pursuing the development of treatments with novel mechanisms of action in addition to new formulation types, both independently as well as through alliances with other companies, Eisai will make further contributions to addressing the diversified needs of and increasing the benefits provided to Alzheimer's disease patients and their families as well as healthcare professionals.
[ Please refer to the following notes for further information on BAN2401, and BioArctic Neuroscience ]
Media Inquiries:
Public Relations Department,
Eisai Co., Ltd.
+81-(0)3-3817-5120
< Notes to editors >
1. About BAN2401
Current research suggests that an intermediate (Aβ protofibril) in the aggregation process of the amyloid-β peptide (Aβ) initiates and drives the neurodegenerative process in Alzheimer's disease. These Aβ protofibrils have been proven to be toxic elements of the disease in a number of studies conducted by Prof. Lars Lannfelt of Uppsala University. BioArctic Neuroscience has been working in collaboration with Eisai to develop antibodies that can specifically recognize these toxic Aβ protofibrils. BAN2401 is a humanized monoclonal antibody that selectively recognizes and eliminates Aβ protofibrils.
Eisai obtained the global rights from BioArctic Neuroscience to study, develop, manufacture and market BAN2401 for the treatment of Alzheimer's disease. However, BioArctic Neuroscience retains the rights to market the antibody in Nordic countries.
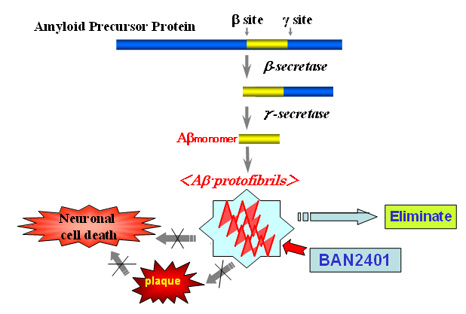
2. About BioArctic Neuroscience
BioArctic Neuroscience is a biopharmaceutical company with a proprietary technology for developing therapeutic monoclonal antibodies for the treatment of Alzheimer's disease, Parkinson's disease, and other disorders caused by misfolded proteins.
For more information on BioArctic Neuroscience, please visit www.bioarctic.se
