November 18, 2013
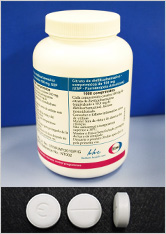
In October 2013, Eisai began its free supply of diethylcarbamazine citrate (DEC) 100 mg tablets produced at its Vizag Plant in India to the World Health Organization (WHO) to help treat and eliminate lymphatic filariasis worldwide. The DEC tablets will be provided to WHO over a period of seven years and are expected to benefit some 250 million people living in at-risk communities through WHO's mass drug administration programs in 26 countries endemic for the disease.
High-quality DEC tablets are currently in short supply in endemic countries and this poses a major obstacle for the effective elimination of lymphatic filariasis. As part of an agreement signed by Eisai and WHO in November 2010, Eisai developed a DEC tablet formulation for worldwide distribution and, after acquiring WHO prequalification in August 2013, has since begun production of its “Eisai-original” DEC tablets to be shipped to targeted endemic countries. The first destinations confirmed to receive Eisai's initial DEC shipment are the Pacific nations of Papua New Guinea, Kiribati, Tuvalu and Fiji. Approximately 6.25 million people* are reported to be living in at-risk communities in these countries alone.
-
*WHO Preventive Chemotherapy and Transmission Control (PCT) Databank (as of 2012)

