Policy and Basic Concept
“The quality of every single tablet, capsule and ampule that we produce is integral to the life of the patient.”
This is Eisai's general policy on product quality, with each employee profoundly aware that every medicine supplied by Eisai is directly linked to patients and people's lives. It is this conviction that is reflected in every aspect of our production activities, and we believe that as long as there are patients and the people around the world in need of our medicine, there exists a mission and a responsibility to ensure the stable supply of high-quality pharmaceutical products. To achieve this, Eisai is consistently striving for high quality through a robust management system that oversees all processes from research on drug substances and formulations to production and distribution.
Targets and Actions
The scope of quality assurance for pharmaceutical products extends even to the delivery and actual use by patients and the people who need our products.
Eisai thinks that our materiality is the pursuit and realization of customer quality that satisfies the tacit expectations of patients and the people, as well as meeting the regulations, standards and regulatory dossiers in each country. In our daily operations, we aim to supply all products with the same level of quality regardless of manufacturing sites; our own sites or Contract Manufacturing Organizations (CMOs) so that our products can be utilized with confidence in any country and region of the world.
Under the global Quality Assurance Structure, we implement manufacturing and quality control in accordance with globally unified Good Manufacturing Practice (GMP: regulations for production and quality management), while placing emphasis on maintaining product quality in the distribution phase based on Good Distribution Practice (GDP: standards for proper distribution) in order to supply pharmaceutical products to patients with the quality just as manufactured. Global project teams discuss Eisai’s policies and procedures, especially for the strengthening of data integrity (completeness, consistency, accuracy of data) to ensure the reliability of data which is essential to assure the quality of products, and for GDP to manage product quality in an increasingly complex supply chain.
Another area of focus is the smooth transition from development to commercial production by further strengthening collaboration with the R&D department. This includes proactive involvement in development activities towards the commercial launch from the early stages before new drug application is submitted, as well as checking consistency of the submission documents for post approval compliance with laws and regulations. Eisai contributes to the realization of social good through these initiatives in order to deliver our products as quickly as possible to patients and the people who are waiting for them.
Structures
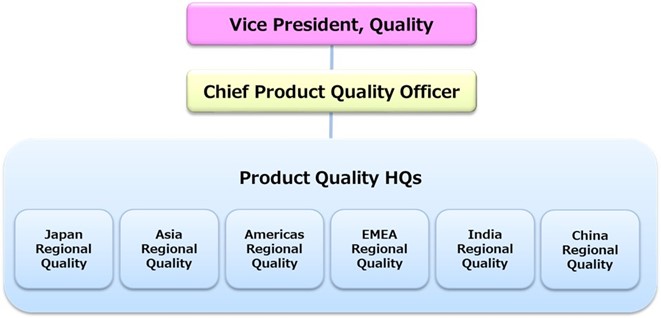
Under the lead of the Vice President of Quality who is responsible for Quality Assurance (QA) of pharmaceutical products, Chief Product Quality Officer has been appointed and a system responsible for ensuring the quality of all Eisai products marketed globally has been established. The heart of Eisai QA is the Global Product Quality Committee (the GPQC), which is comprised of the Executive Senior Director of Product Quality HQs and the six regional quality heads in Japan, Asia, Americas, EMEA (Europe, Middle East and Africa), India and China.
The GPQC collects product quality and regulatory information with global impact from each region, and members discuss and make decisions on how to verify and respond to such information.
In addition to the QA departments at Eisai manufacturing sites in Japan, the United States, the UK, China, India and Indonesia, Eisai has established a structure that can rapidly respond to events which happen in each region and country, assigning Quality Assurance Managers at each Eisai marketing affiliate to deal with complaint handling, manage changes in regulatory approvals, and recall procedures.
Initiatives
Eisai has built a system to ensure quality that can be utilized with confidence in any country and region of the world.
Management of Manufacturing Sites
Our manufacturing sites are evaluated and improved continually by using common Key Performance Indicators (KPIs) and by sharing Corrective Actions/Preventive Actions to improve quality events based on a globally consistent policy.
For our manufacturing sites and Contract Manufacturing Organizations (CMOs), in the event of a quality event, it is shared with others concerned so that similar quality events can be prevented. In addition, periodic GMP audits are implemented for quality management systems of CMOs, and KPIs are determined for CMO management, which enable us to evaluate and improve continually.
Data Reliability
Ensuring integrity of research data and production data, etc. is extremely important for pharmaceutical companies because it is the evidence that provides the basis of product safety and reliability. Failure to ensure data integrity could result in loss of trust in company’ as well as a significant impact on business performance, including delays and discontinuations of new drug development, product recalls, and suspension of sales which would considerably affect patients and the people.
We regularly conduct training to further reinforce data integrity, including the importance of complying with Good Manufacturing Practice (GMP) standards for manufacturing and quality control of medicines, and to behave in accordance with our corporate concept. We are striving to foster an organizational culture of bottom-up communication and an open workplace climate. Further, we have established a system of checks by separating the governance of the manufacturing department from that of the quality control and quality assurance departments. For our Contract Manufacturing Organizations, we conduct regular on site GMP audits as much as possible, including observing actual data acquisition procedures/processes at the site and verifying records as one of the items in our audits.
Further, especially at our manufacturing sites, we have been automating the calculation and recording of important data so that data integrity can be strengthened and that the sites can transform to next generation plants that are able to be managed remotely.
Compliance to Approved Documents
Medicines can only be delivered to patients when they meet regulatory requirements of each country, and when they are manufactured and tested in accordance with approved methods and the intended quality is secured. At Eisai, in addition to periodically checking for consistency with approval documents, we also conduct self-inspection of quality control and quality assurance systems, and risk assessments that take into account quality issues at other companies, ensuring that quality events, if they occur, are detected and our quality assurance system is continually improved. In addition, we strive to standardize the appropriate manufacturing and analytical procedures through further strengthening collaboration with the R&D Department, such as by confirming in pre-submission stage that preparations for commercial manufacturing (manufacturing and analytical methods, etc.) are in line with the submission documents. These activities enable us to contribute to smooth transition to commercial production then to product launches as early as possible after NDA approval.
Initiatives against Counterfeit Drugs
As the globalization of the development and distribution of pharmaceutical products accelerates remarkably, there have been reports of an increasing risk of counterfeit drugs not only in developing countries and emerging countries but also in developed countries. In addition to our regular quality-assurance activities, Eisai is implementing global product security activities through a specialist organization to ensure that our products are delivered to patients safely and securely.
In collaboration with regulatory authorities, other companies in the industry and industry associations, Eisai is actively participating in monitoring and establishing measures against counterfeit drugs and the illegal distribution of drugs. When a case is detected, Eisai responds quickly by undertaking investigations, taking various legal steps and assuring stable supplies, with these efforts led by the Product Security Execution Committee.
We have also taken appropriate measures to address the recent increase in the distribution of counterfeit pharmaceuticals through social media.
To prevent counterfeit drugs and illegally distributed drugs from reaching the front-lines of medical care, it is necessary to more accurately ascertain the actual state of drug distribution and ensure the proper and safe supply and use of pharmaceuticals. For these reasons, initiatives to ensure the traceability of pharmaceutical distribution are being implemented in regions worldwide and some countries and regions have already established regulations.
Eisai uses special packaging and printing techniques to make illegitimate products harder to make and easier to detect, and closely monitors internet and social media to identify and take action against suspicious product sources. We are also proactively participating in these types of initiatives promoted by regulatory authorities, pharmaceutical companies and the front-lines of medical care. Furthermore, training has been conducted by Product Security externally to educate law enforcement, regulatory agencies and industry on Eisai products and initiatives.
